Abstract
Introduction
Psoriasis is a chronic inflammatory condition that can significantly impact the quality of life (QoL), regardless of the level of skin involvement. Apremilast is indicated for the treatment of moderate to severe psoriasis. Real-world data regarding the impact of apremilast on patient-reported outcomes in clinical practice in the Netherlands is lacking.
Methods
The prospective, multicenter observational Apremilast in Real-Life Psoriasis Treatment (APRIL) study enrolled patients ≥ 18 years old with moderate to severe plaque psoriasis receiving apremilast in clinical practice in the Netherlands. Patients were followed-up for 12 months, with assessments scheduled at 6 and 12 months. The primary outcome was Dermatology Life Quality Index (DLQI) response (score ≤ 5 or ≥ 5-point improvement from baseline) at 6 months. Secondary patient-reported outcomes included EQ-5D and skin-specific parameters; exploratory outcomes were Patient Benefit Index (PBI) and Work Productivity and Activity Impairment (WPAI).
Results
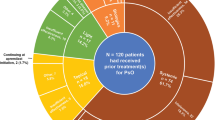
Of the 155 patients enrolled (February 2016–June 2019), 153 received apremilast; 69 (45%) and 39 (26%) continued treatment at 6 and 12 months, respectively. Psoriasis in special areas was common (scalp, 65%; nail, 51%; palmoplantar, 27%). Most patients (92%) had received prior systemic antipsoriatic therapies. Of the 151 patients with a baseline DLQI value, 56 (37%) achieved DLQI response at 6 months. Mean (standard deviation) PBI scores were 3.5 (1.2) and 3.8 (1.1) at 6 and 12 months, respectively. Improvements in DLQI, EQ-5D, and WPAI scores and disease signs and symptoms, including itch and special areas, were observed at 6 and 12 months. Adverse events were consistent with the known safety profile.
Conclusions
In the Netherlands, patients with moderate to severe psoriasis receiving apremilast for up to 12 months reported improved disease-related QoL, skin involvement, and patient-reported outcomes. These data add to the growing body of evidence demonstrating apremilast is an effective treatment for psoriasis, itch, and special areas (scalp and palms).
Trial Registration
ClinicalTrials.gov, NCT02652494.





Similar content being viewed by others
Data Availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at http://www.amgen.com/datasharing.
References
Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590.
Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871–81.
Augustin M, Reich K, Glaeske G, Schaefer I, Radtke M. Co-morbidity and age-related prevalence of psoriasis: analysis of health insurance data in Germany. Acta Derm Venereol. 2010;90:147–51.
Kleyn CE, Talbot PS, Mehta NN, et al. Psoriasis and mental health workshop report: exploring the links between psychosocial factors, psoriasis, neuroinflammation and cardiovascular disease risk. Acta Derm Venereol. 2020;100:adv00020.
Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18-23 (discussion ii4-5).
Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-65.e19.
Sampogna F, Tabolli S, Abeni D. Living with psoriasis: prevalence of shame, anger, worry, and problems in daily activities and social life. Acta Derm Venereol. 2012;92:299–303.
Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10.
Papadavid E, Rompoti N, Theodoropoulos K, Kokkalis G, Rigopoulos D. Real-world data on the efficacy and safety of apremilast in patients with moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol. 2018;23:1173–9.
Ohata C, Ohyama B, Kuwahara F, Katayama E, Nakama T. Real-world data on the efficacy and safety of apremilast in Japanese patients with plaque psoriasis. J Dermatolog Treat. 2019;30:383–6.
Shah BJ, Mistry D, Chaudhary N, Shah S. Real-world efficacy and safety of apremilast monotherapy in the management of moderate-to-severe psoriasis. Indian Dermatol Online J. 2020;11:51–7.
Del Alcázar E, Suárez-Pérez JA, Armesto S, et al. Real-world effectiveness and safety of apremilast in psoriasis at 52 weeks: a retrospective, observational, multicentre study by the Spanish Psoriasis Group. J Eur Acad Dermatol Venereol. 2020;34:2821–9.
Augustin M, Kleyn CE, Conrad C, et al. Characteristics and outcomes of patients treated with apremilast in the real world: results from the APPRECIATE study. J Eur Acad Dermatol Venereol. 2021;35:123–34.
Reich K, Korge B, Magnolo N, et al. Quality-of-life outcomes, effectiveness and tolerability of apremilast in patients with plaque psoriasis and routine German dermatology care: results from LAPIS-PSO. Dermatol Ther. 2022;12(1):203–21.
Ghislain PD, Lambert J, Hoai XL, et al. Real-life effectiveness of apremilast for the treatment of psoriasis in Belgium: results from the observational OTELO study. Adv Ther. 2022;39:1068–80.
Klein TM, Blome C, Kleyn CE, et al. Real-world experience of patient-relevant benefits and treatment satisfaction with apremilast in patients with psoriasis: an analysis of the APPRECIATE study. Dermatol Ther. 2022;12:81–95.
Ioannides D, Antonakopoulos N, Chasapi V, et al. A real-world, non-interventional, prospective study of the effectiveness and safety of apremilast in bio-naïve adults with moderate plaque psoriasis treated in the routine care in Greece—the ‘APRAISAL’ study. J Eur Acad Dermatol Venereol. 2022;36:2055–63.
Otezla [summary of product characteristics]. Breda, the Netherlands: Amgen Europe B.V.; July 21, 2020.
Volume 9A of The Rules Governing Medicinal Products in the European Union. Guidelines on Pharmacovigilance for Medicinal Products for Human Use: European Commission; 2008 [September 2008]. https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-9_en#:~:text=Volume%209%20of%20%22The%20rules,both%20human%20and%20veterinary%20use. Accessed 15 Dec 2023.
Foundation for the Code for Pharmaceutical Advertising Code of Conduct for Pharmaceutical Advertising: The Foundation for the Code for Pharmaceutical Advertising; 2019 [1 July 2019]. https://www.cgr.nl/en-GB/Gedragscode-Geneesmiddelenreclame/Code. Accessed 15 Dec 2023.
Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159:997–1035.
Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43.
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–65.
Augustin M, Radtke MA, Zschocke I, et al. The patient benefit index: a novel approach in patient-defined outcomes measurement for skin diseases. Arch Dermatol Res. 2009;301:561–71.
Feuerhahn J, Blome C, Radtke M, Augustin M. Validation of the patient benefit index for the assessment of patient-relevant benefit in the treatment of psoriasis. Arch Dermatol Res. 2012;304:433–41.
Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73:37–49.
Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387–99.
Golbari NM, van der Walt JM, Blauvelt A, Ryan C, van de Kerkhof P, Kimball AB. Psoriasis severity: commonly used clinical thresholds may not adequately convey patient impact. J Eur Acad Dermatol Venereol. 2021;35:417–21.
Cetkovská P, Dediol I, Šola M, et al. Apremilast use in severe psoriasis: real-world data from Central and Eastern Europe. Adv Ther. 2023;40:1787–802.
Giofrè C, Fabbrocini G, Potenza C, et al. Real-world apremilast use for treatment of plaque psoriasis in Italy: patient perspective, characteristics, and clinical outcomes from the DARWIN Study. Adv Ther. 2023;40:3021–37.
Jonak C, Göttfried I, Perl-Convalexius S, et al. Characteristics and outcomes of patients with psoriasis treated with apremilast in the real-world in Austria—results the APPRECIATE study. Ther Adv Chronic Dis. 2023;14:20406223231152784.
Mease PJ, Hatemi G, Paris M, et al. Apremilast long-term safety up to 5 years from 15 pooled randomized, placebo-controlled studies of psoriasis, psoriatic arthritis, and Behçet's syndrome. Am J Clin Dermatol. 2023;24(5):809–20.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance.
Writing support was funded by Amgen Inc. and provided by Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company. Editorial support was provided by Claire Desborough, Amgen UK.
Funding
This study was sponsored by Celgene; Amgen Inc. acquired the worldwide rights to Otezla (apremilast) on November 21, 2019. Amgen B.V. funded the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Conceptualization: Elke M.G.J. de Jong. Methodology: Juul van den Reek, Elke M.G.J. de Jong. Data collection: Juul M.P.A. van den Reek, Robert J.T. van der Leest, Ruud Prevoo, Elke M.G.J. de Jong. Data analysis and investigation: Sarah E. Thomas, Elke M.G.J. de Jong. Data interpretation: All authors. Writing – original draft preparation: Sarah E. Thomas. Writing – review and editing: Juul van den Reek, All authors.
Corresponding author
Ethics declarations
Conflict of interest
Juul M.P.A. van den Reek: Carried out clinical trials for AbbVie, Celgene, Almirall, and Janssen; has received speaking fees/attended advisory boards from AbbVie, Janssen, Bristol Myers Squibb, Almirall, LEO Pharma, Novartis, UCB, and Eli Lilly; received reimbursement for attending or chairing a symposium from Janssen, Pfizer, Celgene, and AbbVie. All funding is not personal but goes to the independent research fund of the Department of Dermatology, Radboud University Medical Centre (Radboudumc) Nijmegen, the Netherlands. Robert J.T. van der Leest: Nothing to disclose. Sarah E. Thomas: Carries out clinical trials for Janssen and Novartis. All funding is not personal but goes to the independent research fund of the Department of Dermatology, Radboud University Medical Centre (Radboudumc) Nijmegen, the Netherlands. Ruud Prevoo: Nothing to disclose. Margreet E. Plantenga: Employee of and stockholder in Amgen B.V. Elke M.G.J. de Jong: Received research grants for the independent research fund of the Department of Dermatology of the Radboud University Medical Center Nijmegen, the Netherlands from AbbVie, Bristol Myers Squibb, Janssen Pharmaceutica, LEO Pharma, Lilly, Novartis, and UCB; ZonMw, KCE, NWO, the National Psoriasis Foundation USA, RUMC/VGZ and RUMC/SMK. Has been a consultant, paid speaker, and/or participated in research for AbbVie, Amgen, Almirall, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Galapagos, Janssen Pharmaceutica, Lilly, Novartis, LEO Pharma, Sanofi, and UCB. All funding is not personal but goes to the Radboud University Medical Centre (Radboudumc), Nijmegen, the Netherlands.
Ethical Approval
This study was approved by the Code Geneesmiddelenreclame (CGR) and performed in accordance with Volume 9A of the Rules Governing Medicinal Products in the European Union, the Gedragscode Geneesmiddelenreclame of the CGR, and good pharmacovigilance practice. The study was approved by a national central ethics committee for observational studies in the Netherlands. nWMO statement given by the Regionale Toetsingscommissie Patiëntgebonden Onderzoek (RTPO) located in Leeuwarden, Netherlands, Ref. no. RTPO 959. Dutch Clinical Research Foundation (DCRF) METC* 2015–119. All patients provided written informed consent before study initiation. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Additional information
Prior Presentation: Interim analyses from the APRIL study were presented in a poster at EADV 2018, AAD 2019, and NVED 2020.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van den Reek, J.M.P.A., van der Leest, R.J.T., Thomas, S.E. et al. Improved Quality of Life in Patients with Psoriasis Receiving Apremilast: Real-World Data from the Netherlands. Adv Ther 41, 1594–1605 (2024). https://doi.org/10.1007/s12325-023-02759-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02759-9