Abstract
Introduction
Over the course of 2022, numerous key clinical trials with valuable contributions to clinical cardiology were published or presented at major international conferences. This review seeks to summarise these trials and to reflect on their clinical context.
Methods
The authors reviewed clinical trials presented at major cardiology conferences during 2022, including the American College of Cardiology (ACC), European Association for Percutaneous Cardiovascular Interventions (EuroPCR), European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), American Heart Association (AHA), European Heart Rhythm Association (EHRA), Society for Cardiovascular Angiography and Interventions (SCAI), TVT-The Heart Summit (TVT) and Cardiovascular Research Technologies (CRT). Trials with a broad relevance to the cardiology community and those with potential to change current practice were included.
Results
A total of 93 key cardiology clinical trials were identified for inclusion. Interventional cardiology data included trials evaluating the use of new generation novel stent technology and new intravascular physiology strategies such as quantitative flow ratio (QFR) to guide revascularisation in stable and unstable coronary artery disease. New trials in acute coronary syndromes and intervention focused on long-term outcomes of optimal medical therapy (OMT), revascularisation in ischaemic dysfunction and left main (LM) intervention. Structural intervention trials included latest data on optimal timing and anticoagulation strategies in transcatheter aortic valve replacement (TAVR), in addition to expanding evidence in mitral and tricuspid valve interventions. Heart failure data included trials with sodium-glucose cotransporter 2 (SGLT2) inhibitors, iron replacement and novel drugs such as omecamtiv. Prevention trials included new data on proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and polypill strategies. In electrophysiology, new data regarding optimal timing of ablative therapy for atrial fibrillation (AF) in addition to novel screening strategies were evaluated.
Conclusion
This article presents a summary of key clinical cardiology trials published and presented during the past year and should be of interest to both practising clinicians and researchers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A concise summary of over 90 key cardiology trial presented at major international conferences during 2022. | |
Clinically relevant trials with potential to impact and change current practice. | |
Updates across the spectrum of cardiology including interventional and structural, acute coronary syndromes, antiplatelet therapies, electrophysiology, atrial fibrillation, preventative therapies, and heart failure. |
Introduction
In 2022, multiple clinical trials with the potential to influence current practice and future guidelines were presented at major international meetings including the American College of Cardiology (ACC), European Association for Percutaneous Cardiovascular Interventions (EuroPCR), European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), American Heart Association (AHA), European Heart Rhythm Association (EHRA), Society for Cardiovascular Angiography and Interventions (SCAI), TVT-The Heart Summit (TVT) and Cardiovascular Research Technologies (CRT). In this article, we review key studies across the spectrum of cardiovascular subspecialties including acute coronary syndromes (ACS), interventional and structural, electrophysiology and atrial fibrillation, heart failure and preventative cardiology.
Methods
The results of clinical trials presented at major international cardiology meetings in 2022 were reviewed. In addition to this, a literature search of PubMed, Medline, Cochrane library and Embase was completed, including the terms “acute coronary syndrome”, “atrial fibrillation”, “coronary prevention”, “electrophysiology”, “heart failure” and “interventional cardiology”. Trials were selected based on their relevance to the cardiology community and the potential to change future clinical guidelines or guide further phase 3 research. This article is based on previously completed work and does not involve any new studies of human or animal subjects performed by any of the authors.
Advances in Percutaneous Coronary Intervention
Several practice changing trials in Percutaneous Coronary Intervention (PCI) have been published this year (Table 1). Historically, PCI has been used to treat ischaemic cardiomyopathy, despite limited supporting evidence [1]. In the REVascularisation for Ischaemic VEntricular Dysfunction (REVIVED-BCIS2) trial [2], 700 patients with left ventricular ejection fraction (LVEF) ≤ 35% and extensive coronary artery disease (CAD), as defined by the British Cardiovascular Intervention Society (BCIS) jeopardy score, were randomised to PCI or optimal medical therapy (OMT). Over a median follow-up time of 3.4 years, PCI versus OMT alone did not result reduction in the primary composite outcome of death or hospitalization for heart failure [37.2% vs. 38.0%; HR 0.99; 95% confidence interval (CI), 0.78–1.27; P = 0.96] [2].
The optimal treatment for left main (LM) and multivessel CAD remains hotly debated. New observational data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) [3] compared outcomes among 10,254 such patients undergoing PCI (52.6%) versus coronary artery bypass grafting (CABG) (47.4%). PCI was associated with a 59% increased risk of death versus CABG after 7 years of follow-up (P = 0.011). Despite the limitations of observational data, findings are in keeping with the NOBLE study [4], supporting use of CABG where clinically appropriate in LM patients with additional multivessel CAD.
In contrast, a meta-analysis of 2913 patients from four RCTs (SYNTAXES, PRECOMBAT, LE MANS, and MASS II) undergoing PCI versus CABG for LM or multivessel CAD [5] did not report any significant difference in 10-year survival (RR 1.05; 95% CI 0.86–1.28), nor significant difference in the subgroup with LM disease alone or multivessel disease alone. This may reflect a lower extent of non-LM disease complexity in the four trials.
Of note, a new analysis from the SYNergy Between PCI With TAXUS and Cardiac Surgery Extended Study (SYNTAXES) evaluated mortality according to presence or absence of bifurcation lesions [6]. In the PCI group, those undergoing stenting of ≥ 1 bifurcation lesions versus no bifurcation stenting, had a higher risk of death at 10 years (30.1% vs. 19.8%; P < 0.001). Furthermore, a 2 versus 1 stent bifurcation strategy was associated with a higher risk of death at 10 years (HR 1.51; 95% CI 1.06–2.14). Conversely, in the CABG, the presence or absence of bifurcation lesions had no impact on mortality. As this was a post hoc analysis, results can only be considered hypothesis-generating, but are in keeping with previous data highlighting the complexity of bifurcations and the preference for a simple rather than a complex strategy where possible.
Female sex has been associated with worse outcomes following PCI related to smaller vessel disease. However, previous LM have been unclear and, given that LM has a larger diameter, more equivalent results. A substudy of the NOBLE trial [7] showed no difference in outcomes for male versus female, with both showing an excess of major adverse cardiovascular and cerebrovascular events (MACCE) with PCI at 5 years, although no difference in all-cause mortality.
For those undergoing PCI for LM disease, the IDEAL-LM (Individualizing Dual Antiplatelet Therapy After Percutaneous Coronary Intervention in patients with left main stem disease) study [8] reported that a strategy of short 4-month DAPT (dual-antiplatelet therapy) plus a biodegradable polymer platinum-chromium everolimus-eluting stent was non-inferior to a strategy of conventional 12-month DAPT plus durable polymer cobalt-chromium everolimus-eluting stent (DP-CoCr-EES), with respect to a composite of death, MI or target vessel revascularisation at 2 years. However, the shorter DAPT strategy did not show any reduction in bleeding events.
The Complete Revascularization with Multivessel PCI for Myocardial Infarction (COMPLETE) trial previously reported that complete versus culprit-only PCI had lower risk of cardiovascular (CV) death/myocardial infarction (MI) over 3 years of follow-up. In a new pre-specified analysis [9], complete versus culprit-only PCI was associated with a greater absence of residual angina (87.5% vs. 84.3%; P = 0.013) and improved quality of life, as assessed via the 19-item Seattle Angina Questionnaire, including reduced physical limitation.
Improving PCI outcomes in patients with diabetes remains a focus of several trials. The Second-generation drUg-elutinG Stents in diAbetes: a Randomized Trial (SUGAR trial), which randomised 1175 patients with diabetes and CAD to an amphilimus-eluting stent (Cre8 EVO) vs. conventional Resolute Onyx stent, previously reported that the Cre8 stent met non-inferiority and was associated with a possible 35% reduction in Target Lesion Failure (TLF) at 12 months [10]. However, by 2 years [11], the difference in TLF was no longer significant (10.4% vs. 12.1%; HR 0.84; 95% CI 0.60–1.19) with numerical but non-significant differences in the individual components of cardiac death (3.1% vs. 3.4%), target vessel MI (6.6% vs. 7.6%), and target lesion revascularization (4.3% vs. 4.6%). While these 2-year results were disappointing, we await results of further studies of new stents in this clinical setting, including the ABILITY trial (NCT04236609) comparing an Abluminus DES + sirolimus-eluting stent system versus Xience.
Quantitative flow ratio (QFR), an angiography-based approach to estimate the fractional flow reserve, previously reported superiority versus conventional angiography guidance at 1 year in the FAVOR III (Comparison of Quantitative Flow Ratio Guided and Angiography-Guided Percutaneous InterVention in Patients With cORonary Artery Disease) trial [1]. New data report that the benefit with the QFR-guided strategy was sustained at 2 years, associated with a 34% reduction in the composite of death, MI or ischaemia-driven revascularization [8.5% vs. 12.5%; HR 0.66 (95% CI 0.54–0.81)] [12]. The degree of outcome improvement was greatest amongst those patients in whom the pre-planned PCI strategy was modified by QFR.
Current ESC guidelines give post-PCI surveillance with stress testing with a Class IIb recommendation. The POST-PCI (Routine Functional Testing or Standard Care in High-Risk Patients after PCI) trial randomised 1706 patients at 1 year after PCI to routine functional testing (nuclear stress testing, exercise electrocardiography, or stress echocardiography) versus standard care [13]. Use of routine functional testing failed to show any reduction in the primary outcome of death MI, or hospitalization for unstable angina at 2 years (5.5% vs. 6.0%; HR, 0.90; 95% CI 0.61–1.35; P = 0.62), supporting standard care in these patients.
Procedural time in graft-angiography studies may be much longer than a non-graft cases. The Randomised Controlled Trial to Assess Whether Computed Tomography Cardiac Angiography Can Improve Invasive Coronary Angiography in Bypass Surgery Patients (BYPASS CTCA), randomised 688 prior CABG patients to CTCA prior to coronary angiography versus standard care. Those who underwent prior CTCA had a shorter procedure duration (mean 17.4 vs. 39.5 min; OR − 22.12; 95% CI − 24.68 to − 19.56), less contrast during the invasive angiogram (mean 77.4 vs. 173 mls), less contrast-induced nephropathy (3.2% vs. 27.9%; P < 0.0001) and 40% greater patient satisfaction [14]. BYPASS CTCA thus supports consideration of prior CTCA particularly with more complex or uncertain graft location or patients at greater renal risk.
The 2018 ESC guidelines recommend radial access for PCI unless overriding procedural considerations. A new patient-level meta-analysis of 7 trials, incorporating 21,700 patients reported that, at 30 days, transradial versus transfemoral access was associated with a 23% reduction in all-cause mortality (1.6% vs. 2.1%; P = 0.012) and 45% reduction in major bleeding (1.5% vs. 2.7%; P < 0.001) [15]. However, transradial access is not without complications, the commonest of which is radial artery occlusion. In the RIVARAD (Prevention of Radial Artery Occlusion With Rivaroxaban After Transradial Coronary Procedures) trial, 538 patients were randomised following coronary angiography to rivaroxaban 10 mg once daily for 7 days versus standard care (no rivaroxaban) [16]. At 30 days, use of rivaroxaban was associated with a 50% reduction in radial artery occlusion as defined by ultrasound (6.9% vs. 13.0%; OR 0.50; 95% CI 0.27–0.91). Bleeding Academic Research Consortium (BARC)-defined bleeding events were numerically but not significantly higher in the rivaroxaban group (2.7% vs. 1.9%; OR 1.4; 95% CI 0.4–4.5). To assess whether distal radial artery puncture might reduce occlusion rates, the Distal Versus Conventional Radial Access DISCO-RADIAL) trial randomised 1,307 patients to distal versus conventional radial access [17]. Distal access was associated with shorter median hemostasis time (153 vs. 180; P < 0.001), but radial artery spasm was more common (5.4% vs. 2.7%; P = 0.015), crossover rates were higher (7.4% vs. 3.5%; P = 0.002) and no difference in the primary endpoint of occlusion on vascular ultrasound was noted at discharge (0.31% vs. 0.91%; P = 0.29).
While radial access now considered preferable, transfemoral access is still required in certain cases. As transfemoral operator skills may potentially decline through reduction in volume or lack of experience, ultrasound-guided access techniques are increasingly being used. The UNIVERSAL (Routine Ultrasound Guidance for Vascular Access for Cardiac Procedures) trial randomised 621 patients to femoral access with ultrasound guidance and fluoroscopy versus fluoroscopy alone [18]. Interestingly, and in contrast with previous trials, ultrasound guidance was not associated with significant reduction in the composite of BARC 2, 3, and 5 bleeding or major vascular complication at 30 days (12.9% vs. 16.1%; p = 0.25).
The strategy of multi-arterial CABG is endorsed by surgical guidelines but takes longer, is more technically demanding and can be associated with increased complications, such as deep sternal wound infections. An observational single-centre study by Momin et al. of 2979 patients undergoing isolated CABG (from 1999 to 2020) [19] reported those receiving total arterial revascularization had the longest mean survival (18.7 years) versus single internal mammary artery (SIMA) plus vein grafts 16.1 years; P < 0.00001) versus vein grafts only (10.4 years; P < 0.00001). Interestingly, survival with total arterial revascularization was not significantly different to SIMA plus radial artery ± vein grafting (18.60 years). This study supports the durability of arterial grafting, although conclusions are limited by its non-randomised design. Conversely, Saadat et al. stratified 241,548 patients from the Society of Thoracic Surgeons (STS) database undergoing isolated CABG in 2017 [20] into 3 groups: single arterial (86%), bilateral internal thoracic artery-multi-arterial (BITA-MABG; 5.6%), and radial artery multiarterial (RA-MABG; 8.5%). After risk adjustment, the observed to expected event (O/E) ratios showed no significant difference in mortality between the three strategies (1.00 vs. 0.98 vs. 0.96) and the risk of deep sternal wound infection was highest in the BITA-MABG group (1.91 vs. 0.90 vs. 0.96). Given the ongoing data uncertainty, results from the prospective randomised ROMA trial are eagerly awaited (NCT03217006).
Structural: Aortic Valve Interventions
There has been a dramatic expansion in transcatheter aortic valve interventions over the past decade [1]. A recent analysis of US registry data conducted by Sharma et al. reported a near doubling in transcatheter aortic valve replacement (TAVR) volume overall between 2015 and 2021 (44.9% vs. 2021, 88%, P < 0.01), including a 2.7 fold increase in those < 65 years (now similar to surgical aortic valve replacement (SAVR) (47.5% TAVR vs. 52.5% SAVR, P = ns) particularly in younger patients with heart failure (HF) (OR 3.84; 95% CI 3.56–4.13; P < 0.0001), or prior CABG (OR, 3.49; 95% CI, 2.98–4.08; P < 0.001) [21]. These numbers may further increase across all risk categories with the early long-term data from the seminal PARTNER (Placement of AoRTic TraNscathetER Valve Trial) trials awaited.
Emerging evidence from trials such as AVATAR (Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis) and RECOVERY (Early Surgery Versus Conventional Treatment in Very Severe Aortic Stenosis) suggests that early intervention for severe aortic stenosis (AS), before patients develop symptoms, may be of benefit [1]. In a pooled analysis of key trials (PARTNER2A, 2B &3) involving 1974 patients (mean age 81 years; 45% women), Généreux et al. evaluated the relationship between cardiac damage at baseline and prognosis in patients with severe symptomatic AS who underwent AVR (40% SAVR, 60% TAVI) [22]. Baseline cardiac damage was defined using a 0–4 scoring system (0 = no damage and 4 = biventricular failure). Baseline damage correlating strongly with 2-year mortality (HR 1.51 per higher stage; 95% CI 1.32–1.72) with each increase in stage conferred a 24% increase in mortality (P = 0.001) (from stage 0 = 2.5% to stage 4 = 28.2%) suggesting a role for earlier intervention. Several ongoing trials, such as EARLY TAVR (Evaluation of TAVR Compared to Surveillance for Patients With Asymptomatic Severe Aortic Stenosis), TAVR UNLOAD (Transcatheter Aortic Valve Replacement to UNload the Left Ventricle in Patients With ADvanced Heart Failure) and PROGRESS (Management of Moderate Aortic Stenosis by Clinical Surveillance or TAVR), aim to answer these questions directly.
Valve in valve (VIV) TAVR is being increasing utilised in patients with failed AVR; however, it remains unclear whether these patients do better with or without balloon valve fracture (BVF). In a registry analysis of 2975 patients undergoing VIV-TAVR (with balloon-expandable SAPIEN 3 or SAPIEN 3 Ultra) between December 2020 and March 2022, Garcia et al. [23] reported that BVF versus no BVF led to larger mean valve area (1.6 vs. 1.4 cm2; P < 0.01) and lower mean valve gradient (18.2 vs. 22.0 mm Hg; P < 0.01) but also to higher rates of death or life-threatening bleeding (OR 2.55; 95% CI 1.44–4.50) and vascular complications (OR 2.06; 95% CI 0.95–4.44). However, sub-analysis suggested the increase in mortality was mainly if BVF undertaken before VIV-TAVR (OR 2.90; 95% CI 1.21–6.94), whereas no difference was noted if undertaken after VIV-TAVR. This suggests that VIV-BVF should only be performed once the operator has a new TAVR in place.
While designed primarily for AS, conventional TAVR devices have sometimes utilised for the treatment of severe aortic regurgitation (AR). The novel trilogy heart valve system, specifically developed for AR, and was evaluated in 45 patients (mean age 77, 40% female, mean Euroscore 7.1%) with moderate to severe AR by Tamm et al. [24]. The primary endpoint, a reduction in ≥ 1 AR grade, was met in 100% of cases. There were no episodes of stroke, death, or conversion to open surgery, but 9 patients (23%) required permanent pacing.
Subclinical leaflet thrombosis (SLT) is a relatively common complication of TAVR; however, the optimal treatment strategies, whether with anticoagulation or antiplatelets, remain contested. The multicentre ADAPT TAVR (Edoxaban vs. DAPT in reducing subclinical leaflet thrombosis and Cerebral Thromboembolism After TAVR) randomised 229 patients (mean age 80.1 years; 41.9% men) undergoing TAVR for symptomatic severe AS, and without other indication for OAC, to edoxaban 60 mg or 30 mg once daily versus DAPT with aspirin and clopidogrel [25]. At 6 months, Edoxaban, by intention to treat analysis, was associated with a trend to reduced SLT as assessed by cardiac CT (9.8% vs. 18.4%; P = 0.076) and, in contrast to prior trials with DOAC post-TAVR, there was no difference in bleeding rates (11.7% vs. 12.7%; P = ns). Interestingly, a secondary per-protocol analysis focusing on patients with high compliance did reach statistical significance (19.1% vs. 9.1%; risk ratio 0.48; 95% CI 0.23–0.99). However, despite the use of serial brain MRI, there was no difference in the presence/number of cerebral lesions and no difference in neurocognitive outcomes including stroke at 6 months.
Giustino et al. reported a new secondary analysis from the GALILEO trial (Rivaroxaban-based Antithrombotic Strategy to an Antiplatelet-based Strategy After TAVR to Optimize Clinical Outcomes) which, as described previously [[4]], had randomised 1644 patients post-TAVR without an indication for oral anticoagulation (OAC) to rivaroxaban 10 mg plus aspirin versus DAPT with aspirin plus clopidogrel for 90 days, but was stopped early due to higher thromboembolic bleeding and mortality events in the Rivaroxaban group [26]. In the new analysis, thromboembolic events appeared to be associated with higher risk of mortality (HR 8.41; 95% CI 5.10–13.87) versus BARC 3 bleeding (HR 4.34; 95% CI 2.31–8.15). Furthermore, this mortality risk appeared higher than that conferred by known risk factors such as age (adjusted HR 1.04; 95% CI 1.01–1.08) and chronic obstructive pulmonary disease (COPD) (adjusted HR 2.11; 95% CI 1.30–3.41).
These findings along with previous data from ALANTIS (AntiThrombotic Strategy After Trans-Aortic Valve Implantation for Aortic Stenosis) and ENVISAGE-TAVI AF (Edoxaban Compared to Standard Care After Heart Valve Replacement Using a Catheter in Patients With Atrial Fibrillation) show how the role of DOACs post-TAVI remains uncertain [1]. However, given the devastating impact of thromboembolic events in this patient group, ongoing research is warranted. The absence of a bleeding signal with DOAC in ADAPT TAVR, in which most received lower dose Edoxaban, suggests that lower dose DOAC for a short duration while the valve is endothelialising may improve the risk/benefit ratio.
Another area of current contention is the use of cerebral embolic protection (CEP) to reduce risk of stroke. While current guidance does not mandate use, some operators use in high-risk cases [27]. Kaur et al. conducted a meta-analysis of 1,016 patients (mean age 81.3 years) from several randomised trials (DEFLECT III, MISTRAL-C, CLEAN-TAVI, SENTINEL, and REFLECT I and II) evaluating the TriGuard (Keystone Heart) and Sentinel devices versus standard care. At 30 days, CEP was not associated with a reduction in the primary outcome of all-cause stroke (RR 0.93; 95% CI 0.57–1.53), nor a reduction in mortality. Subsequently, the PROTECTED TAVR (Stroke PROTECTion With SEntinel During Transcatheter Aortic Valve Replacement) trial randomised 300 patients (mean age 72 years, 40% female) to CEP with a Sentinel device versus standard care [28]. Again, no significant difference in primary outcome of stroke at 72 h was noted (2.4% vs. 2.9%, P = 0.30), although numbers were relatively small. The results of BHF PROTECT TAVI (British Heart Foundation Randomised Clinical Trial of Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation) plans to enrol 7000 patients and findings are eagerly awaited.
Stuctural: Mitral and Tricuspid Valve interventions
The favourable findings in COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation [MR) helped lead to device approval [1]. However, it has been suggested the reason COAPT was favourable was the strict eligibility criteria, mandating LVEF ≥ 20% to ≤ 50%, left ventricular end-systolic dimension (LVESD) ≤ 70 mm and failure of aggressive medical therapy [4].
EXPAND (A Contemporary, Prospective Study Evaluating Real-world Experience of Performance and Safety for the Next Generation of MitraClip Devices) [29] was a prospective multicentre registry of 1,041 patients with site-reported MR 3 + /4 + were enrolled and received the MitraClip. A recent analysis compared 125 “COAPT-like” patients meeting COAPT inclusion criteria versus 128 “non-COAPT” patients. At 1 year, COAPT-like patients did not show any difference in the primary outcome of all-cause mortality (22.6% vs. 19.6%, P = 0.37) or heart failure hospitalisation (32.6% vs. 25%, P = 0.08). In keeping with their lower baseline MR severity, more non-COAPT patients achieved reduction in MR to mild or less (≤ 1 +) (97.2% vs. 86.5%), suggesting that Mitraclip may benefit patients beyond the strict COAPT criteria, but prospective randomised data are needed, such as the ongoing EVOLVE-MR (MitraClip for the Treatment of Moderate Functional Mitral Regurgitation).
Previous data from CLASP (Edwards PASCAL TrAnScatheter Mitral Valve RePair System Study) and CLASPII have validated the safety and efficacy of the Edwards PASCAL™ transcatheter valve repair system [1]. CLASP IID randomised 180 patients with severe degenerative symptomatic MR not eligible for surgery (mean age 81 years, 67% male, median STS 5.9%) to transcatheter Edge-to-Edge Repair (TEER) with the Pascal device (Edwards Lifesciences) vs. MitraClip (Abbott) device [30]. At 30 days, the Pascal device met criteria for non-inferiority with respect to the composite endpoint of CV death, stroke, MI, renal replacement therapy, severe bleeding and re-intervention (3.4% vs. 4.8%; P for noninferiority < 0.05). Of interest, the proportion of patients with MR ≤ 1 + was durable in the PASCAL group (87.2% discharge vs. 83.7% at 6 months; P = 0.317); whereas MitraClip outcomes showed some loss of efficacy (88.5% discharge vs. 71.2% at 6 months; P = 0.003). Although only interim data, this hints that the Pascal device may have superior durability.
ViV-transcatheter mitral valve replacement (ViV-TMRV) may be utilised in very high-risk patients without a surgical option on a case-by-case basis despite paucity of real-world outcome data. Bresica et al. retrospectively compared outcomes of 48 patients with bioprosthetic mitral valve (MV) failure undergoing ViV-TMRV (mean age 65 years, 63% female, mean STS 7.9%) versus 36 patients undergoing re-do MV surgery (mean age 58, 72% female, mean STS 7.1%) [31]. ViV-TMVR was not associated with improvement in 1-year survival (90% vs. 80%, P = 0.33) and was associated with higher average postprocedural gradient (8.9 vs. 5.7 mm Hg; P < 0.001). Thus, ViV-TMRV is a good option for high-risk patients, but in less comorbid patients may not provide as good a long-term benefit as surgery, particularly in those with smaller original surgical valves. Data to come from the ongoing PARTNER 3 (Mitral Valve-in-Valve trial) will be useful to help guide decision-making in such patients.
Several seminal trials, such as TRILUMMINATE (Abbott Transcatheter lip Repair System in Patients With Moderate or Greater TR), Triband (TranscatheterRepair of Tricuspid Regurgitation With Edwards Cardioband TR System Post-Market Study) and TRISCEND (Investigation of Safety and Clinical Efficacy After Replacement of Tricuspid Valve With Transcatheter Device), have led to a much greater focus on transcatheter tricuspid interventions [1].
CLASP TR (Edwards PASCAL Transcatheter Valve Repair System Pivotal Clinical Trial), a prospective single-arm multicentre study, evaluated 1-year outcomes of the PASCAL transcatheter valve repair system in 65 patients (mean age 77 ± 9 years, 55% female, mean STS 7.7%) with severe tricuspid regurgitation (TR) [32]. In keeping with the high baseline comorbidity, major adverse event rate was 16.9% (n = 11) with all-cause mortality 10.8% (n = 7) and 18.5% (n = 12) re-admitted with heart failure. Paired analysis demonstrated significant improvements in New-York Heart Association (NYHA) grade (P < 0.001), KCCQ score (P < 0.001) and 6-min walk test (6MWT) (P = 0.014). Importantly, the reduction in TR severity noted at 30 days (P < 0.001) was maintained at 1 year (100% had ≥ 1 grade reduction and 75% had ≥ 2 grade reduction, P < 0.001).
TRICLASP (Transcatheter Repair of Tricuspid Regurgitation With Edwards PASCAL Transcatheter Valve Repair System), a prospective, single-arm multicentre trial, evaluated 30-day outcomes in 67 of 74 patients (mean age 80 years, 58% female, mean STS 9%) undergoing the Pascal Ace transcatheter repair system for severe symptomatic inoperable TR [33] (Fig. 1). The primary composite outcome of major adverse events was 3% with 88% achieving ≤ 1 grade reduction in TR vs. baseline; P < 0.001), along with significant improvements in NYHA, KCCQ score, and 6MWT (P < 0.001). Longer term follow-up data are awaited.
TriClip-Bright (An Observational Real-world Study Evaluating Severe Tricuspid Regurgitation Patients Treated With the Abbott TriClip™ Device) study [34], a multicentre, prospective study reported 30-day outcomes for 300 patients (78 ± 7.6 years) undergoing the Triclip Transcatheter valve repair system (Fig. 2). The primary endpoint of procedural success (survival to discharge) was met in 91%. Significant reductions in both NYHA and KCCQ score were noted at (P < 0.001). The trial is still actively recruiting, with a planned follow-up duration of 1 year.
Structural: Catheter Based Left Atrial Appendage and Patent Foramen Ovale Closure
While definitive studies to guide patent foramen ovale (PFO) closure practice are still lacking, a multidisciplinary consensus statement by SCAI was published this year [35] recommending closure in patients aged 18–60 with a PFO-associated stroke, platypnoea-orthodeoxia syndrome with no other cause, and systemic embolism with no other cause. Of note in the absence of PFO-associated stroke, the guidance does not recommend PFO closure in transient ischaemic attack, AF with ischaemic stroke, migraine, decompression illness or thrombophilia.
Several left atrial appendage closure (LAAC) devices have been approved in recent years with favourable long-term data published last year [1] for the Watchman LAAC device (Boston Scientific). The AMULET IDE trial (Amplatzer Amulet Left Atrial Appendage Occluder Versus Watchman Device for Stroke Prophylaxis) [36] trial randomised patients with non-valvular atrial fibrillation (AF), not suitable as anticoagulation to LAAC with an Amulet device (n = 934) versus Watchman device (n = 944). At 3 years, there was no difference in the primary composite endpoint of CV mortality, ischaemic stroke or systemic embolism (11.1% vs. 12.7%, P = 0.31) all-cause mortality (14.6% vs. 17.9%; P = 0.07) or major bleeding (16.1% vs. 14.7%; P = 0.46). Similarly, updated data from the US LAAC registry, comparing the Watchman FLX to its previous iteration, the Watchman 2.5, was published this year by Freeman et al. [37] who reported US LAAC registry outcomes from 54,206 patients (mean age 76 years; 59% men) undergoing LAAC with the new Watchman FLX (n = 27,103) versus previous Watchman 2.5 (n = 27,103). In-hospital major adverse events were significantly lower with the new Watchman FLX (1.35% vs. 2.4%, OR 0.57: 95% CI 0.50–0.65) driven by reductions in pericardial effusion requiring intervention (0.42% vs. 1.23%), device embolization (0.02 vs. 0.06%) and major bleeding (1.08% vs. 2.05%). Longer follow-up will help clarify if technical aspects between devices confer long-term clinical outcome advantages.
Despite the evolution of device technology for LAAC, key clinical questions, such as anticoagulation strategy, remain. Freeman et al. conducted a US LAAC registry analysis of 31,994 patients who underwent Watchman LAAC between 2016 and 2018. Only 12.2% of patients received the full anticoagulation protocol mandated by clinical trials [38] (Fig. 3). In contrast to previous European reports from EWOLUTION (Registry on WATCHMAN Outcomes in Real-Life Utilization), the 45-day adjusted adverse event rate was longer if discharged on warfarin alone (HR 0.692; 95% CI 0.569–0.841) or DOAC alone (HR 0.731; 95% CI 0.574–0.930) versus warfarin plus aspirin, suggesting that further research is needed to guide the optimal antithrombotic strategy post-LAAC.
Post-Watchman implantation antithrombotic strategies and associated risk of adverse outcomes. The most common strategy was warfarin plus aspirin. The lowest risk of adverse events was seen in groups anticoagulated with either warfarin or NOAC alone (primarily driven by reduced bleeding rates). Interestingly, there was no difference in ischaemia stroke or device-related thrombus between groups. Reproduced with the kind permission of the Journal of the American College of Cardiology [38]
Acute Coronary Syndromes
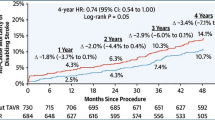
The ISCHAEMIA trial (Initial Invasive or Conservative Strategy for Stable Coronary Disease) was a previously reported that routine invasive therapy versus optimal medical therapy (OMT) in stable patients with moderate ischaemia did not reduce major adverse events (MAE), but the possibility of excess events over longer follow-up was queried. The ISCHAEMIA-EXTEND study (median follow-up 5.7 years) [39] reported that while there was still no difference in all-cause mortality in routine invasive versus medical therapy (12.7% vs. 13.4%, P = 0.74), after 2 years the survival curves for cardiovascular (CV) death started to diverge and by 7 years were significantly lower in the routine invasive group (6.4% vs. 8.6% HR 0.78; 95% CI 0.63, 0.96). Conversely, there was an increase in non-CV death in the routine invasive group (5.5% vs. 4.4%, HR 1.44; 95% CI 1.08–1.91). On balance, this still supports an initial OMT strategy but highlights the utility of understanding anatomy to risk stratify and perhaps identify those patients who will benefit the most from CV risk reduction (Fig. 4). Ten-year follow-up data will prove informative.
Kaplan–Meier survival Curves demonstrating cumulative event rate of all-cause mortality and CV mortality at 8 years in the ISCHAEMIA-EXTEND trial. Notably, a lower 7-year rate CV mortality was demonstrated in the invasive group [6.4% vs. 8.6%; adjusted hazard ratio, 0.78 (95% CI, 0.63–0.96)]; however, there was no difference in all-cause mortality [7-year rate, 12.7% in invasive strategy, 13.4% in conservative strategy; adjusted hazard ratio, 1.00 (95% CI, 0.85–1.18)] [39]. Reproduced with the kind permission of Circulation
New onset, stable chest pain remains a substantial burden on healthcare systems. SCOT-HEART (Scottish COmputed Tomography of the HEART Trial) and PROMISE (PROspective Multicenter Imaging Study for Evaluation of Chest Pain) previously reported benefit in early computed tomography coronary angiogram (CTCA) for the evaluation of stable chest pain[40]. FFR-CT may further improve CT diagnosis. PRECISE (Prospective Randomized Trial of the Optimal Evaluation of Cardiac Symptoms and Revascularization) [41] randomised 2103 patients (mean age 58 years, 50% women) with suspected CAD to a risk scoring algorithm (with low-risk patients deferred and high-risk patients undergoing FFR-CT) versus standard care. At a median follow-up of 11.8 months, algorithm-guided use of FFR-CT resulted in markedly lower MACE (4.2% vs. 11.3%; adjusted HR 0.29; 95% CI 0.20–0.41), driven by a lower rate of catheterisation (4.2% vs. 11.3%; adjusted HR 0.29; 95% CI 0.20–0.41). There was no difference in all-cause death. A subsequent cost-effectiveness analysis is ongoing.
Despite current advances in ACS detection, prediction of recurrent events remains difficult. Batra et al. [42] assessed the predictive valve of biomarker modelling (with hs-TNT, CRP, DGF-15, cystatin C, NT-proBNP) from 14,221 patients enrolled in PLATO (A Comparison of Ticagrelor and Clopidogrel in Patients With Acute Coronary Syndrome) and TRACER (Trial to Assess the Effects of Vorapaxar (SCH 530,348; MK-5348) in Preventing Heart Attack and Stroke in Participants With Acute Coronary trials. An outcome model termed “ABC-ACS Ischaemia” predicted 1-year risk of CV death/MI with C-indices of 0.71 and 0.72 in the development and validation cohorts, respectively. While encouraging, such models likely need to be integrated with additional individual patient characteristics in improve risk prediction.
Optical Coherence Tomography (OCT) has demonstrable utility in assessing plaque morphology and so may be useful in delineating between different aetiologies of ACS. The Tokyo, Kanagawa, Chiba, Shizuoka, and Ibaraki active OCT applications for ACS (TACTICS) registry, evaluated plaque morphology in 702 ACS patients undergoing OCT-guided PCI [43] and reported rupture was the commonest aetiology (59%), followed by plaque erosion (26%), and then calcification (4%) (Fig. 5). However, at 12 months, calcified nodules conferred the worst outcome with a 32.1% MACE rate compared to 12.4% and 6.2% amongst ruptures or erosions, respectively.
Illustration reproduced from TACTICS registry demonstrating OCT (Optical Coherence Tomography) images of the different causes of ACS (Acute Coronary Syndromes) using OCT-defined morphological assessment. Reproduced with kind permission from the Journal of American College of Cardiology [43]
Antiplatelet therapy
Strategies to shorten DAPT duration post-PCI in high bleeding risk patients continue to be evaluated. Longer-term follow-up at 15 months [44] of the MASTER DAPT (Management of High Bleeding Risk Patients Post-Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Prolonged DAPT Regimen) confirmed initial results [1], with the incidence of the composite endpoint (death, MI, stroke, major bleeding) remaining non-inferiority for shortened DAPT versus standard care (HR 0.92, 95% CI 0.76–1.12; P = 0.40), but a significantly lower rate of major bleeding in the short DAPT group (HR 0.68, 95% CI 0.56–0.83; P = 0.001). These data, although important, were applied in the context of contemporary stent design such as the biodegradable-polymer sirolimus-eluting Ultimaster stent (Terumo) as used in MASTER DAPT.
Effective reversal of antiplatelets could be helpful when active bleeding risk outweighs ischaemic risk, particularly in elderly patients. No formal antiplatelets reversal agents are currently licensed; however, an interesting drug under investigation is Bentracimab—a recombinant IgG1 monoclonal antibody antigen-binding fragment that binds with high affinity to ticagrelor and its active metabolite. Bhatt et al., in a phase IIb trial, randomised 205 patients (mean age 61 years, female 50%) already treated with DAPT for 30 days to Bentracimab (n = 154) versus placebo (n = 51). Use of Bentracimab was associated with a significant reduction in the primary endpoint of percentage inhibition of P2Y12 reaction units at 4 h (P < 0.0001) without any excess of thrombotic events or deaths [45]. Further larger-scale phase III trials are eagerly awaited.
In patients with an indication for antiplatelet monotherapy, previous studies have suggested a possible benefit for clopidogrel versus aspirin at least in certain patient subgroups. PANTHER (P2Y12 inhibitor vs. aspirin monotherapy in patients with coronary artery disease) was a meta-analysis of several large, randomised trials totalling 24,325 patients with established coronary artery disease (mean age 64 years, 22% women) which compared P2Y12 inhibition (62% clopidogrel, 38% ticagrelor) versus aspirin [46]. Use of P2Y12 inhibition was associated with a 12% reduction in the primary composite outcome of CV death, MI or stroke at 18 months (5.5% vs. 6.3%; HR 0.88; 95% CI 0.79–0.97) driven by a lower risk of MI (HR 0.77; 95% CI 0.66–0.90), but with no difference in stroke (HR 0.85; 95% CI 0.70–1.02) or bleeding (6.4% vs. 7.2%; HR 0.89; 95% CI 0.81–0.98). While firm conclusions are difficult due to the inclusion of 2 different P2Y12 inhibitors, it suggested P2Y12 inhibitor may be warranted instead of aspirin for long-term secondary prevention in patients with coronary artery disease.
Indobufen is a reversible COX inhibitor with similar anti-thrombotic effects to aspirin but less gastrointestinal side effects and potentially lower risk of bleeding [47]. The OPTION (the Efficacy and Safety of Indobufen and Low-dose Aspirin in Different Regimens of Antiplatelet Therapy) trial randomised 4,551 patients (mean age 61 years; 65% male) without acute troponin rise, undergoing PCI with DES to 1 year of DAPT (indobufen 100 mg BD plus clopidogrel 75 mg; n = 2258 vs. aspirin plus clopidogrel 100 mg OD; n = 2293). At 1 year, use of indobufen versus aspirin meet non-inferiority with respect to the primary composite outcome (CV death, MI, stroke, ISR and BARC type 2,3 or 5 bleeding) (4.47% vs. 6.11%; HR 0.73; 95% CI 0.56–0.94; P < 0.001 for noninferiority). The secondary safety endpoint of BARC 2, 3 or 5 bleeding was lower with indobufen (2.97% vs. 4.71%; HR 0.63; 95% CI 0.46–0.85), driven by a reduction in BARC 2 bleeding (1.68% vs. 3.49%; P < 0.001). These intriguing data suggest a potential new treatment option particularly for patients with gastrointestinal bleeding or aspirin allergy.
Full dose anticoagulation plus antiplatelet therapy significantly increases bleeding risk but the role of low-dose anticoagulation for vascular prevention continues to be studied. Asundexian is a novel oral activated factor XI inhibitor which may lower thromboembolic events but with lower bleeding risk [48]. In the phase II PACIFIC-AMI trial (Study to Gather Information About the Proper Dosing and Safety of the Oral FXIa Inhibitor BAY 2,433,334 in Patients Following an Acute Heart Attack), 1601 patients (median age 68 years, 23% women) with recent acute MI were randomised to asundexian (10 mg, 20 mg or 50 mg) versus placebo in addition to standard DAPT. At 4 weeks, asundexian was not associated with a significant increase in the pre-specified safety outcome of BARC2 bleeding versus placebo 0.98 (90% CI, 0.71–1.35), although there was a numerical increase in bleeding with higher asundexian doses. Based on this trial, asundexian 50 mg daily is being considered for a phase III cardiovascular outcomes trial in acute MI.
Asundexian was also evaluated in the phase IIb PACIFIC-STROKE trial (Study to Gather Information About the Proper Dosing and Safety of the Oral FXIa Inhibitor BAY 2,433,334 in Patients Following an Acute Stroke) which randomised 1808 patients with non-embolic ischaemic stroke to asundexian (10 mg, 20 mg or 50 mg) versus placebo in addition to standard care including antiplatelet therapy [49]. Asundexian (whether by pooled or individual dose analysis) was not associated with reduction in the primary efficacy outcome of ischemic stroke or overt stroke at 6 months, although the primary safety outcome of major significant bleeding was not significantly different [asundexian pooled vs. placebo HR1·57 (90% CI 0·91–2·71)]. It thus remains unclear if asundexian has a useful role in ischaemic stroke.
In current PPCI guidelines, Bivalirudin (Class IIa) was replaced by unfractionated heparin (UFH) (Class I) as previous studies reported equipoise in clinical outcomes but more difficult drug administration with Bivalirudin. BRIGHT-4 (Bivalirudin With Prolonged Full Dose Infusion Versus Heparin Alone During Emergency PCI) randomised 6,016 PPCI patients from 63 Chinese centres in open-label fashion to Bivalirudin bolus plus infusion for a median of 3 h versus UFH bolus [50]. Patients underwent predominantly radial PPCI (93%) without any prior thrombolytic, anticoagulant or glycoprotein inhibitor treatment. At 30 days, Bivalirudin was associated with a 31% reduction in the primary outcome of all-cause or BARC 3–5 bleeding (HR 0.69; 95% CI 0.53–0.91, P = 0.007), reduced BARC 3–5 bleeding (HR 0.21; 95% CI 0.08–0.54), reduced all-cause mortality (3.0% vs. 3.6%, P = 0.04), and reduced stent thrombosis (0.4% vs. 1.1%, P = 0.0015). Despite these favourable data, given the inherent difficulties in bivalirudin delivery and moderate increase in cost versus UFH, it is unclear if BRIGHT-4 findings will change practice, although a stronger guideline recommendation would be expected.
Tongxinluo (TXL) is a traditional Chinese medicine, approved in China for the treatment of stroke and angina [51]. CTS-AMI (China Tongxinluo Study for Myocardial Protection in Patients With Acute Myocardial Infarction) was a randomised trial of 3755 patients with STEMI undergoing PPCI at 124 Chinese centres to TXL versus placebo (in addition to standard therapy). Use of TXL was associated with a 36% reduction in the primary composite outcome of CV death, revascularisation, MI and stroke at 30 days (3.39% vs. 5.25%; RR 0.64; 95% CI 0.47–0.88) and a 30% reduction in cardiac death (2.97% vs. 4.24%; RR 0.70; 95% CI: 0.50–0.99). While the findings are dramatic, further work is necessary to understand the mechanism of action of this novel drug and further randomised multicentre trials to confirm efficacy.
Electrophysiology and Devices
Following on from the HIS-Alternative trial (His Pacing Versus Biventricular Pacing in Symptomatic HF With Left Bundle Branch Block) [1], which reported similar outcomes with His-Bundle CRT (His-CRT) versus conventional biventricular CRT (BiV-CRT), the LBBP-RESYNC (Left Bundle Branch Versus Biventricular Pacing For Cardiac Resynchronization Therapy) trial randomised 40 patients with non-ischaemic cardiomyopathy, LBBB and an indication for resynchronisation to left bundle branch CRT (LBB-CRT) versus standard BiV-CRT pacing [52]. LBB-CRT was associated with a larger improvement in LVEF at 6 months (21.1% vs. 15.6%; P = 0.039, 95% CI 0.3–10.9), greater reduction in LV end systolic volumes and greater reduction NT-proBNP (Fig. 6). Vijayaraman et al. presented a retrospective analysis of 477 patients [53] comparing those who underwent conduction pacing (LBB pacing or His-bundle) versus conventional BiV-CRT. Conduction pacing was associated with a lower incidence of the primary composite of death or heart failure hospitalisation (28.3% vs. 38.4%; P = 0.013), mainly driven by a reduction in HF hospitalisations. Vijayaraman et al. also presented a retrospective analysis of 212 patients with rescue LBB pacing who met indications for CRT but had coronary venous lead failure or were non-responders to BiV-CRT [54]. LBB pacing (successful in 94%) was associated with improvement in LVEF from 29% at baseline to 40% at follow-up (P < 0.001) (Fig. 7). The MELOS (Multicentre European Left Bundle Branch Area Pacing Outcomes Study) registry evaluated 2533 patients from 14 European centres undergoing transseptal left bundle branch area pacing (LBBAP), 27.5% for heart failure and 72.5% for bradycardia [55]. LB fascicular capture was most common (69.5%) followed by LV septal capture (21.5%) then proximal LBB capture (9%). Overall complication rate was 11.7%, including ventricular trans-septal complications in 8.3%. Overall, these trials collectively support the efficacy and safety of conduction system pacing as a suitable alternative to conventional BiV-CRT, although larger randomised trials are required to formally test superiority.
Central illustration from the LBBP-RESYNC trial indicating the randomization process and total numbers in LBBP-CRT group and the BiVP-CRT group. Also bar graph highlighting the change in LVEF (%) from baseline, at 6 months of treatment int the LBBP-CRT group 21.1% vs. 15.6% in the BiVP-CRT group (P = 0.039, 95% CI 0.3–10.9). Reproduced with kind permission from the Journal of the American College of Cardiology [52]
Graphical abstract from Vijayaraman et al. demonstrating the improvements in QRS duration (ms), NYHA class, ejection fraction (%) and end diastolic dimension (mm) in patients receiving rescue LBBBAP in patients with lead failure or non-responders. For all patients, the QRS duration reduced by 31 ms (P < 0.001), ejection fraction increased from 29 to 40% (P < 0.001), NYHA class reduced from between 2.3–3 to < 2 (P < 0.001) and end diastolic diameter reduced from 59 to 56 mm (P < 0.001). Reproduced with kind permission from the Heart Rhythm Journal Ltd [54]
Infections related to cardiac implanted electronic devices (CIEDs) have high mortality and morbidity, and the European heart rhythm association (EHRA) consensus advises prompt extraction [56]. Pokornery et al. analysed a Medicare database of 11,619 patients admitted with a CIED infection [57] of whom only 2,109 (28.2%) had device extraction within 30 days. Device extraction versus no extraction was associated with reduction in 1-year mortality (HR 0.79, 95% CI 0.70–0.81) and early device extraction within 6 days versus no extraction was associated with a 41% reduction in 1-year mortality (P < 0.001).
Subcutaneous ICDs (S-ICDs) have been evaluated in previous trials including PRAETORIAN and UNTOUCHED [58, 59] as an alternative to transvenous systems for patients at risk of lead complications or infections. The ATLAS -ICD (Avoid Transvenous Leads in Appropriate Subjects) trial randomised 593 patients with an indication for ICD to SC-ICD versus transvenous ICD (TV-ICD) implantation [60]. SC-ICD was associated with a 92% reduction in perioperative lead complications at 6 months (0.4% vs. 4.8%; OR 0.08; 95% CI 0.00–0.55), although the composite safety outcome (including the primary outcomes plus device-related infection requiring surgical revision, significant wound hematoma requiring evacuation or interruption of oral anticoagulation, MI, stroke/TIA, or death) was similar (4.4% vs. 5.6%; OR 0.78, 95% CI 0.35–1.75) and inappropriate shocks were non-significantly more common (2.7% vs. 1.7%; HR2.37, 95% CI 0.98–5.77).
In heart failure patients, there is contradictory evidence whether defibrillator capability improves prognosis in patients receiving CRT. RESET-CRT (Re-evaluation of Optimal Re-synchronization Therapy in Patients with Chronic Heart Failure) retrospectively compared outcomes in 847 CRT-P versus 2722 CRT-D patients undergoing CRT (of whom 27% had a non-ischaemic aetiology and exclusion criteria included recent ACS, revascularisation, or any indication for secondary prevention ICD)[61]. The primary endpoint of all-cause mortality at 2.35 years follow-up (adjusted for age and entropy balance) was non-inferior for CRT-P versus CRT-D (HR 0.99, 95% CI 0.81–1.20), suggesting no mortality benefit with defibrillator capability in this population.
Atkas et al. compared propensity matched outcomes of 535 patients with ICD versus 535 patients without ICD from the Empagliflozin arm of the Emperor-Reduced trial [62]. Those with ICD versus no ICD had non-significantly lower mortality (HR 0.74, 95% CI 0.51–1.07, P = 0.114) and sudden cardiac death (HR 0.59, 95% CI 0.31–1.15, P = 0.122). However, despite propensity matching, the results were confounded by differences in medical therapy between groups, with more ICD patients receiving B-blockers and ARNIs but fewer receiving ACE-I/ARBs and MRAs.
Ventricular Arrhythmias and SCD
The VANISH (Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs) trial previously demonstrated superiority with regards to mortality, VT storm and appropriate ICD shocks of catheter ablation versus escalated AAD therapy in patients with previous MI and VT [63]. A new sub-analysis compared shock-treated VT events and appropriate shock burden between the 2 groups. Catheter ablation was associated with a significant reduction in shock-treated VT events (39.07 vs. 64.60 per 100 person-years; HR 0.60; 95% CI 0.38–0.95) and total shock burden (48.35 vs. 78.23; HR 0.61; 95% CI 0.37–0.96).
Prediction risk of sudden cardiac death (SCD) after MI has typically guided by LVEF < 35%, but many patients with LVEF < 35% who receive ICD never require it, whereas some with higher LVEF are still at risk of SCD. The additional predictive value of CMRI, in particular core scar size and grey zone size, for the PROFID risk prediction model was investigators in 2,049 patients imaged > 40 days post-MI [64]. In the subgroup without ICD, use of CMRI data versus no CMRI data significantly improved prediction of SCD [area under curve (AUC) of model 0.753 vs. AUC 0.618]. In the subgroup with ICD, addition of CMRI data did not significantly improve prediction of SCD (AUC 0.598 vs. 0.535). This suggests CMRI may be useful to risk stratify post-MI and guide ICD use but further prospective studies are required.
The SMART-MI-ICM trial [1] previously reported that, in post-MI patients with EF 35–50%, implantable cardiac monitor (ICM) use versus control was associated with higher rates of arrhythmia detection although the clinical significance was unclear. The BIOGUARD-MI (BIO monitorinG in Patients With Preserved Left ventricUlar Function AfteR Diagnosed Myocardial Infarction) trial [65] aimed to assess the clinical value of arrhythmia detection on ICM, by randomising 804 patients with NSTEMI/STEMI to ICM versus standard care. Use of ICM was not associated with an overall significant reduction in the primary composite endpoint of CV death or hospitalisation at 2.5 years (HR 0.84, P = 0.21, 95% CI 0.64–1.10), although a reduction was noted in the NSTEMI subgroup (HR 0.69, 95% CI 0.49–0.98). This subgroup observation can only be hypothesis generating but is plausible given the more complex and co-morbid nature of a NSTEMI population.
Atrial Fibrillation
While smartwatches may improve detection of atrial fibrillation (AF), including asymptomatic AF, previous studies have reported high false positive rates. The mAF-App II trial, which used Huawei smartwatch photoplethysmography, reported data from 2.8 million people in China who downloaded the app [66]. During 4 years follow-up, 12,244 (0.4%) people received a query AF notification, 5,227 attended for clinical evaluation with ECG and 24-h Holter monitoring and, within this group, AF was confirmed in 93.8%. This suggests much better specificity than previous studies, although the notification rate was lower than some studies, reflecting the relatively young population, and clinical data were not available for the 7017 people who received a notification but did not attend for evaluation.
Unlike previous Apple, Fitbit and Huawei studies, E-Brave [67] used the Preventicus smartphone app and invited 67,488 policyholders of a German health insurance scheme to participate, of whom 5,551 met inclusion criteria and agreed to enroll (AF naïve, median age 65 years; 31% female; median CHA2DS2-VASc of 3) and were randomised to active AF screening (photoplethysmogram [PPG] for 1 min twice per day for 2 weeks then twice weekly for 6 months, plus 2-week loop recorder if abnormal PPG) versus standard care. At 6 months, those in the active arm had double the rate of AF detection requiring OAC treatment (1.33 vs. 0.63%; OR 2.12; 95% CI 1.19–3.76). After 6 months, those without a new AF diagnosis were invited to cross-over to the opposite study arm, and, after a further 6 months, active screening with the app again doubled the detection and treatment of AF (1.38% vs. 0.51%; OR 2.75; 95% CI 1.42–5.34). Given the widespread availability of smartphones particularly in higher-risk populations, this may be a useful public health intervention, although further prospective studies are required to evaluate clinical outcomes of treating AF detected in this fashion.
AF has been widely associated with increased risk of dementia and better control of AF may reduce this risk. Zeitler et al. using the Optum Clinformatics database, evaluated the propensity-matched risk of dementia in 19,088 patients following catheter ablation versus 19,088 patents treated with antiarrhythmic drugs (AAD) for AF [68]. Catheter ablation was associated with a 41% reduction in risk of dementia (HR 0.59; 95% CI 0.51–0.68; P < 0.0001) and a 49% reduction in the secondary endpoint of mortality (HR 0.51, 95% CI 0.46–0.55, P < 0.001), supporting the value of effective AF treatment in this population.
The Augustus trial previously reported the benefit of apixaban instead of vitamin-K antagonist (VKA) and ongoing P2Y12i monotherapy rather than DAPT for patients with AF and ACS/PCI [4]. Harskamp et al. undertook a new analysis of 4,386 patients from Augustus to assess if benefits varied depending on baseline HASBLED (≤ 2 vs. ≥ 3) and CHAD2S2VASc (≤ 2 vs. ≥ 3) scores [69]. Apixaban was associated with lower bleeding versus VKA irrespective of baseline risk [HR: 0.57 (HAS-BLED ≤ 2), HR 0.72 (HAS-BLED ≥ 3); interaction P = 0.23] and lower risk of death or hospitalization (HR 0.92 (CHA2DS2-VASc ≤ 2); HR 0.82 (CHA2DS2-VASc ≥ 3); interaction P = 0.53]. Aspirin versus placebo increased bleeding irrespective of baseline risk [HR: 1.86 (HAS-BLED ≤ 2); HR: 1.81 (HAS-BLED ≥ 3); interaction P = 0.88] with no significant difference in death or hospitalization [HR: 1.09 (CHA2DS2-VASc ≤ 2); HR: 1.07 (CHA2DS2-VASc ≥ 3); interaction P = 0.90].
The INVICTUS (Investigation of Rheumatic AF Treatment Using Vitamin K Antagonists, Rivaroxaban or Aspirin Studies) trial [70], randomised 4565 patients with rheumatic mitral valve and at high risk (CHAD2S2VASc ≥ 2, mitral valve area ≤ 2cm2, left atrial spontaneous contrast or thrombus) to Rivaroxaban versus VKA. Rivaroxaban was associated with increased incidence of the primary composite endpoint of stroke, systemic embolus, MI, or death from vascular/unknown cause (560 vs. 446 events; HR 1.25, 95% CI 1.10–1.41) despite suboptimal VKA control (only 33.2% having at appropriate INR enrolment, and the time in therapeutic range (TTR) being only 56–65% during follow-up). Rivaroxaban was also associated with a 37% increased risk of stroke and 23% increased risk. Thus, for AF and rheumatic mitral valve disease, VKA remains preferable to rivaroxaban.
Previous studies reported that high-power, short duration (HPSD) versus conventional radiofrequency ablation (RFA) for AF was more effective with similar safety [1]. The POWER FAST III (High Radiofrequency Power for Faster and Safer Pulmonary Vein Ablation) trial randomised 267 patients with AF to HPSD versus conventional RFA [71]. HPSD was associated with a reduced ablation time but no difference in the primary efficacy outcome of freedom of atrial arrhythmia (99.2% vs. 98.4% in right pulmonary veins, 100% vs. 100% in left pulmonary veins) or the primary safety outcome of oesophageal lesions at endoscopy (7.5% vs. 6.5%; P = 0.94).
Both conventional RFA and cryoablation for pulmonary vein isolation induce injury to neurocardiac structures (nerves and ganglia) which may be detected may release of S100b levels and post-procedure rise in heart rate [72]. The technique of pulsed field ablation (PFA) may reduce neurocardiac trauma. Lemoine et al. randomised 56 patients to PFA versus cryoablation for AF. In those treated with PFA versus cryoablation, troponin I levels were 3 times higher (P < 0.01), indicating more myocardial injury, but S100b levels were 2.9 times lower (P < 0.001), and there was no increase in post-procedural heart rate (vs. marked increase with cryoablation; P < 0.01), indicating less neurocardiac damage with PFA. In addition, procedural success and durability of PFA appears encouraging. Keffer et al. evaluated 41 patients undergoing pulmonary vein PFA [73]. The primary outcome of AF > 30 s or atrial tachycardia after a 30-day blanking period detected on 7-day Holter monitoring at 3 and 6 months occurred in 5 patients, of whom 3 underwent redo ablation during which all pulmonary veins were found to be still isolated.
EAST-AFNET 4 previously reported a benefit of early rhythm control versus standard care in patients with AF [1], but there has been a paucity of data regarding initial ablation in such patients. In PROGRESSIVE-AF (a 3-year follow-up of the EARLY-AF trial), 303 patients with newly diagnosed symptomatic paroxysmal AF were randomised to upfront ablation versus AAD [74]. Ablation was associated with a 75% reduction in the primary outcome of progression to persistent AF/flutter/tachycardia requiring cardioversion (1.9% vs. 7.4%; HR 0.25; 95% CI 0.09–0.70), a 49% reduction in any atrial arrhythmia > 30 s (56.5% vs. 77.2%; HR 0.51; 95% CI 0.38–0.67), a 69% reduction in hospitalisations (5.2% vs. 16.8%; RR 0.31; 95% CI 0.14–0.66) and 53% reduction in adverse effects (11% vs. 23.5%; RR 0.47; 95% CI 0.28–0.79).
Use of botulinum toxin A to reduce AF was assessed in the NOVA (NeurOtoxin for the PreVention of Post-Operative Atrial Fibrillation) study which randomised 323 patients undergoing cardiac (bypass and/or valve) surgery to epicardial botulinum toxin A (125 units or 250 units) versus placebo [75]. Overall, botulinum 125 units or 250 units versus placebo was not associated with a reduction in the primary outcome of AF > 30 s at 30 days (RR 0.80; 95% CI 0.58–1.10 and RR 1.04; 95% CI 0.79–1.37), respectively, although in the patient subgroup > 65 years, botulinum 125 units was associated with AF reduction (RR 0.64; 95% CI 0.43–0.94) which may be considered hypothesis-generating and warrant further study.
Etripamil is a novel non-dihydropyridine calcium channel blocker, which may be given as a nasal spray, for acute treatment of patients with paroxysmal supraventricular tachycardia (PSVT) or AF. The RAPID (Efficacy and Safety of Etripamil for the Termination of Spontaneous PSVT) study [76] screened 706 patients with PSVT ultimately assigning in random fashion 135 patients to etripamil versus 120 to placebo. Etripamil was associated with more than double the primary outcome of conversion to sinus rhythm within 30 min (64.3% vs. 31.2%; HR 2.62; 95% CI 1.66–4.15) and a median time to conversion of 17 min (almost 3 times quicker than placebo).
Heart Failure
Previous studies have shown the selective cardiac myosin activator Omecamtiv Mecarbilon may improve CV outcomes in HFrEF patients [1, 76]. To assess functional impact, the METEORIC-HF (Effect of Omecamtiv Mecarbil on Exercise Capacity in Chronic Heart Failure With Reduced Ejection Fraction) trial [78] randomised 276 patients with LVEF ≤ 35%; NYHA II-III (in 2:1 fashion) to Omecamtiv Mecarbilon versus placebo for 20 weeks, in addition to standard therapy. Surprisingly, despite good tolerability and the previous favourable CV outcome data, Omecamtiv Mecarbilon was not found to improve exercise capacity (assessed by peak oxygen uptake on cardiopulmonary exercise stress testing).
A major stumbling block in optimising HF medications can be hyperkalaemia. Patiromer, a non-absorbed sodium-free potassium-binding polymer increases faecal potassium excretion. The DIAMOND (Patiromer for the Management of Hyperkalemia in Subjects Receiving RAASi for HFrEF) trial [79] randomised 1642 patients with HFrEF and renin–angiotensin–aldosterone system inhibitor (RAASi)-related hyperkalaemia to Patiromer versus placebo. Over a period of 13–42 (mean 27) weeks, Patiromer was associated with less increase in potassium (adjusted mean change + 0.03 vs. + 0.13 mmol/l; 95% CI –0.13 to 0.07; P < 0.001). The risk of hyperkalamia and need for reduction of MRA dose were numerically (although not statistically) lower. These important findings support Patiromer being incorporated in local HF protocols.
Implementation of HF guidelines can be hampered by many factors. PROMPT-HF (PRagmatic trial of Messaging to Providers about Treatment of Heart Failure) [80] randomised 1310 patients with HFrEF, not already taking all four pillars of therapy to a strategy of targeted, tailored electronic healthcare record alerts to optimise guideline-directed medical therapy (GDMT) versus standard care. The electronic alert strategy was associated with a significant increase in the number of drug classes prescribed at 30 days (26% vs. 19%; adjusted RR 1.41; 95% CI: 1.03–1.93; P = 0.03; number needed to alert = 14).
In an impressive attempt to improve secondary prevention therapy delivery, the SECURE (Secondary Prevention of Cardiovascular Disease in the Elderly Trial) trial [81] randomised 2499 patients with MI ≤ 6 months to an open label polypill, comprising aspirin 100 mg, ramipril (2.5, 5 or 10 mg) and atorvastatin (20 or 40 mg), versus standard care. At 3-year follow-up, use of the polypill was associated with a 24% reduction in the primary endpoint of CV death, type 1 MI or ischaemic stroke (9.5% vs. 12.7%; HR 0.76, 95% CI: 0.6–0.96; P = 0.02).
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) trials continue to dominate HF research. A meta-analysis of 13 SGLT2i trials involving 90,413 participants (82 reported a 37% reduction in risk of progressive renal dysfunction 37% (RR 0·63, 95% CI 0·58–0·69) and a 23% reduction in risk of CV death or HF hospitalisation (RR 0·77; 0·74–0·81). Effects were similar in diabetics versus non-diabetics and regardless of baseline renal function (Fig. 8).
Primary efficacy outcome and components demonstrated in the EMPULSE trial using the stratified win ratio. Overall, a win ratio of 1.36 was found in favour of empagliflozin (95% CI: 1.09–1.68, P = 0.0054) [84]. Reproduced with the kind permission of the Nature publishing group
When first introduced and before reno-protective properties became clear, SGLT2i use was restricted to patients with eGFR > 60 to optimise glycaemic control. EMPA-KIDNEY (Study of Heart and Kidney Protection With Empagliflozin) [83] randomised 6609 patients with impaired renal function (eGFR 20 to < 45, or eGFR 45 to < 90 plus urinary albumin-to-creatinine ratio > 200) to empagliflozin versus placebo. At 2 years, empagliflozin was associated with a 28% reduction in the primary endpoint of progression of kidney disease (defined as end-stage kidney disease, eGFR < 10, decrease in eGFR ≥ 40% from baseline, death from renal causes) or CV death (13.1% vs. 16.9% of the control group (HR 0.72; 95% CI: 0.64–0.82; P < 0.001).
The EMPULSE (Empagliflozin in Patients Hospitalized for Acute Heart Failure) trial [84] randomised 530 acutely decompensated patients hospitalised with HF, regardless of ejection fraction or diabetic status to Empagliflozin versus placebo. Those with IV vasodilators, IV inotropes, requiring increasing IV diuretic doses, cardiogenic shock or recent ACS were excluded. Empagliflozin versus placebo was more frequently associated clinical benefit in the primary composite endpoint of death, number of HF events, time to first HF event, and change in Kansas City Cardiomyopathy Questionnaire-Total Symptom Score at 90 days (stratified win ratio 1.36; 95% CI 1.09–1.68; P = 0.0054) (Fig. 8).
The DELIVER (Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction) study [85] randomised 6263 hospitalised or recently hospitalised patients with HF and LVEF > 40% to dapagliflozin versus placebo. Dapagliflozin was associated with an 18% reduction in the primary endpoint of death or worsening HF (16.4% vs. 19.5%; HR 0.82, 95% CI 0.73–0.92; P < 0.001).
Acetazolamide, a carbonic anhydrase inhibitor, through reduction of proximal tubular sodium reabsorption, may improve the efficiency of loop diuretics, potentially leading to faster decongestion in patients with acute decompensated heart failure. The ADVOR (Acetazolamide in Decompensated Heart Failure with Volume Overload) study [86] randomised 519 patients with decompensated HF patients to IV acetazolamide (500 mg daily) versus placebo in addition to IV loop diuretics (at twice the oral maintenance dose) examining the role. Acetazolamide was associated with a 46% improvement in attaining the primary endpoint of absence of signs of fluid overload at 3 days (42.2% vs. 30.5%; RR 1.46, 95% CI 1.17–1.82; P < 0.001) with higher urine output and natriuresis but without an excess of acute kidney injury, hypokalaemia, or hypotension.
While the importance of optimised dosing of HF treatment is well established, since HF therapies may be associated with hypotension and renal decline, the ideal rate of titration is less clear. The STRONG-HF (Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing, of Heart Failure Therapies) trial [87] randomised 1078 patients admitted to hospital with acute HF to rapid up-titration (achieving full recommended doses within 2 weeks of discharge) versus usual care. Rapid up-titration was associated with a significantly lower rate of readmission for HF or all-cause death (15.2% vs. 23.3%; 95% CI 2.9–13.2; P = 0.0021), approximately a 10% increase in adverse events, but a similar rate for serious adverse events.
IV iron has a Class IIa recommendation for patients with HF and anaemia. Most trials have used ferric carboxymaltose. IRONMAN (Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK) [88] randomised 1,137 patients with chronic HF and iron deficiency (LVEF < 45%, with Transferrin saturation < 20% or ferritin < 100 µg/l) to ferric derisomaltose (which can be given as a rapid, high-dose infusion) versus usual care. At a median fgollow up of 2.7 years, ferric derisomaltose showed a trend to reduction in the primary composite endpoint of HF hospitalisation and CV death (336 vs. 411 events; RR 0.82, 95% CI 0.66–1.02; P = 0.07) and a significant reduction in HF hospitalisations. Since study outcomes may have been confounded by the COVID-19 pandemic, a pre-specified analysis censoring follow-up on September 30, 2020 was undertaken which reported a significant reduction in the primary endpoint (210 vs. 280 events; RR 0·76 [95% CI 0·58 to 1·00]; P = 0·047).
Myosin inhibition using mavacamten in patients with obstructive hypertrophic cardiomyopathy was examined in the VALOR-HCM (Mavacamten in Adults With Symptomatic Obstructive HCM Who Are Eligible for Septal Reduction Therapy) trial [89] which randomised 112 patients eligible for septal reduction therapy (SRT) to mavacamten (starting at 5 mg and titrating using LVEF and LVOT gradient) versus placebo. After 16 weeks follow-up, mavacamten was associated with marked reduction in obstructive parameters with only 17.9% still meeting guideline criteria for SRT (vs. 76.8% of placebo patients; 95% CI: 0.44–0.74; P < 0.001).
Prevention
Lipoprotein[Lp] (a) is highly genetically determined and higher levels are associated with an increased risk of CV disease. Statins have minimal effect and PCSK9i only modest effect but Olpasiran, a small interfering RNA (siRNA) may enable significant Lp(a) reduction. In the OCEAN(a)-DOSE TIMI 67 trial [90], 281 patients with elevated Lp(a) > 150 nmol/L were randomised to 1 of 4 olpasiran doses (10 mg, 75 mg, or 225 mg every 12 weeks, or 225 mg every 24 weeks) versus placebo. By 36 weeks, the 4 doses of olpasiran were associated with placebo-adjusted percent reductions in Lp(a) concentration of 70.5%, 97.4%, 101.1%, and 100.5%, respectively, along with useful reductions in low-density lipoprotein (LDL) cholesterol and apolipoprotein B. In addition to Olpasiran, other siRNA drugs are in development including SLN360, and pelacarsen, an mRNA-based antisense oligonucleotide targeting the Lp(a) gene being studied in the 8000-patient outcomes study, Lp(a)HORIZON which will hopefully clarify if reduction of Lp(a) is of benefit [91].
Perceived myalgia remains an important limitation for statin adherence. The Cholesterol Treatment Trialists’ Collaboration evaluated incidence of myalgia in a meta-analysis [92] of 19 double-blind trials of statin versus placebo (n = 123,940) and four double-blind trials of more versus less intensive statin regimen (n = 30,724). For the 19 placebo-controlled trials, statin use was associated with a 3% increase in reported muscle pain or weakness at a median 4·3 years follow-up (27.1% vs. 26.6%; RR 1.03, CI 95% 1.01–1.06), but the excess was mainly during the first year, when statin use was associated with an absolute excess of 11 events per 1000 person-years. Similarly, a small increase in reported muscle pain or weakness was seen with higher versus lower intensity statin groups, (36.1% vs. 34.8%; RR 1.05, CI 95% 1.01–1.09). In summary, while statin therapy can cause myalgia, most (> 90%) reports of muscle symptoms by participants allocated statin therapy were not due to the statin.
The FOURIER-OLE (Fourier Open-label Extension Study in Subjects With Clinically Evident Cardiovascular Disease in Selected European Countries) [93] evaluated the long-term follow-up of the FOURIER study in 6635 patients randomised to the PCSK9 inhibitor Evolocumab versus placebo. At a median of 5 years, Evolocumab was associated with resulted in a 20% reduction in CV death, MI or stroke (HR 0.8, 95% CI 0.68–0.93; P = 0.003) with low risk of adverse events.
Elevated uric acid is recognised as an independent risk factor for CV events. The ALL-HEART (Allopurinol versus usual care in UK patients with ischaemic heart disease) study [94] randomised 5721 patients > 60 years with ischaemic heart disease but no history of gout to allopurinol (up-titrated to maximum of 600 mg) versus placebo. However, over a mean of 4.8 years follow-up, allopurinol was not associated with reduction in the primary endpoint of CV death, MI or stroke (11% vs. 11.3%; P = 0.65).
The endothelin pathway has been implicated in the pathogenesis of hypertension, but is currently not targeted therapeutically, leaving this pathway unopposed with currently available drugs. The global PRECISION (Dual endothelin antagonist aprocitentan for resistant hypertension) trial [95] randomised 730 patients with hypertension resistant to at least 3 antihypertensives to the dual endothelin receptor antagonist aprocitentan aprocitentan 12·5 mg or 25 mg versus placebo in a 1:1:1 fashion. At 4 weeks, aprocitentan was associated with met the primary endpoint with greater systolic blood pressure reduction (mean change for aprocitentan 12.5 mg of − 15.3 mmHg and for aprocitentan 25 mg of − 15.2 mmHg vs. placebo − 11.5 mg; P < 0.005 for both treatment doses).
Delivering healthcare in rural environments can be challenging. In China, non-physician village doctors may initiate and titrate antihypertensive medications according to a standard protocol with supervision from primary care physicians, and undertake health coaching on home blood pressure monitoring, lifestyle changes, and medication adherence. The China Rural Hypertension Control Project randomised 33,995 patients from 326 villages to village doctor-led multifaceted intervention versus usual care [96]. By 36 months, the intervention group reported a drop in mean systolic pressure from 157 to 126.1 mmHg, whereas the usual-care group only dropped from 155.4 mmHg to 146.7 mmHg and a significant reduction in the primary composite CV endpoint (1.98% vs. 2.85% per year; HR 0.69, CI 95% 0.63–0.76) with 33% fewer strokes (P < 0.0001), 39% fewer cases of HF (P = 0.005), 24% fewer CV deaths (P = 0.0004), and 15% fewer all-cause deaths (P = 0.009).
Previous trial data [4] suggested a protective effect for nocturnal dosing of anti-hypertensive therapies on cardiovascular events, although the trial methodology was subsequently questioned [97]. The TIME (Treatment in Morning versus Evening) trial randomised 21,104 patients (mean age 65 years, female 43%) to evening versus morning dosing of their regular antihypertensive agent [98]. After 5 years, the primary outcome (composite of vascular death, MI or stroke) occurred in 3.4% of the evening dosing group versus 3.7% of the morning group (P = 0.53). There was no difference in rates of stroke between groups (1.2% vs. 1.3%, P = 0.54); however, there was a modestly higher rate of falls in the morning dosing group (22.2% vs. 21.1%, P = 0.048). This informative trial demonstrates no difference in cardiovascular outcomes with respect to timing of anti-hypertensive dosing albeit a slightly reduced risk of falls with evening dosing.
Limitations
While all summarised trials have been presented at major cardiology conferences in 2022, not all trials have been published as yet in peer-reviewed journals.
Conclusion
This paper has highlighted and summarised the key cardiology trials that were published and presented during 2022. Many will guide clinical practice and influence guideline development. Others have shown encouraging early data which will guide future study.
References
Savage P, Cox B, Linden K, Coburn J, Shahmohammadi M, Menown I. Advances in clinical cardiology 2021: a summary of key clinical trials. Adv Ther. 2022;39(6):2398–437. https://doi.org/10.1007/s12325-022-02136-y.
Perera D, Clayton T, O'Kane PD, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. 2022;387(15):1351–60. https://doi.org/10.1056/NEJMoa2206606
Omerovic E, on behalf of the SCAAR investigators. Survival after PCI or CABG for left main coronary disease: a report from the Swedish Coronary Angiography and Angioplasty Registry. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Linden K, Mailey J, Kearney A, Menown IBA. Advances in clinical cardiology 2019: a summary of key clinical trials. Adv Ther. 2020;37(6):2620–45. https://doi.org/10.1007/s12325-020-01355-5.
Woodhead T, Matthews CJ, Blaxill JM, et al. Meta-analysis comparing 10-year mortality following percutaneous coronary intervention or coronary artery bypass grafting in left main stem or multivessel coronary artery disease. Am J Cardiol. 2022;174:189–91. https://doi.org/10.1016/j.amjcard.2022.04.004.
Kawashima H, Serruys PW, Hara H, et al. 10-year all-cause mortality following percutaneous or surgical revascularization in patients with heavy calcification. JACC Cardiovasc Interv. 2022;15(2):193–204. https://doi.org/10.1016/j.jcin.2021.10.026.
McEntegart MB, Holm NR, Lindsay MM, Oldroyd KG, Mäkikallio T, Hildick-Smith D, Erglis A, Kellerth T, Davidacius G, Menown IBA, et al. Sex-specific clinical outcomes after treatment of left main coronary artery disease. A NOBLE substudy. J Soc Cardiovasc Angiogr Intervent. 2022;1(4):100338
Van Geuns RJ, Chun-Chin C, McEntegart MB, Merkulov E, Kretov E, Lesiak M, O’Kane P, Hanratty CG, Bressollette E, Silvestri M, Wlodarczak A, Barragan P, Anderson R, Protopopov A, Peace A, Menown IBA, Rocchiccioli P, Onuma Y, Oldroyd KG. Bioabsorbable polymer drug-eluting stents with 4-month dual antiplatelet therapy versus durable polymer drug-eluting stents with 12-month dual antiplatelet therapy in patients with left main coronary artery disease: the IDEAL-LM randomised trial. EuroIntervention. 2022;17(18):1467–76.
Mehta SR, Wang J, Wood DA, et al. Complete revascularization vs. culprit lesion-only percutaneous coronary intervention for angina-related quality of life in patients with ST-segment elevation myocardial infarction: results from the complete randomized clinical trial. JAMA Cardiol. 2022;7(11):1091–9. https://doi.org/10.1001/jamacardio.2022.3032.
Romaguera R, Salinas P, Gomez-Lara J, et al. Amphilimus- vs. zotarolimus-eluting stents in patients with diabetes mellitus and coronary artery disease: the SUGAR trial. Eur Heart J. 2022;43(13):1320–1330. https://doi.org/10.1093/eurheartj/ehab790
Salinas P. Two-year outcomes of the randomized second-generation drug-eluting stents in diabetes (SUGAR) trial. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Song L, Xu B, Tu S, et al. 2-year outcomes of angiographic quantitative flow ratio-guided coronary interventions. J Am Coll Cardiol. 2022;80(22):2089–101. https://doi.org/10.1016/j.jacc.2022.09.007.
Park DW, Kang DY, Ahn JM, et al. Routine functional testing or standard care in high-risk patients after PCI. N Engl J Med. 2022;387(10):905–15. https://doi.org/10.1056/NEJMoa2208335JonesD.
Jones D et al. The BYPASS-CTCA study: a randomised controlled trial assessing the value of computed tomography cardiac angiography (CTCA) in improving patient-related outcomes in patients with prior CABG undergoing invasive coronary angiography. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Gargiulo G, Giacoppo D, Jolly SS, et al. Effects on mortality and major bleeding of radial versus femoral artery access for coronary angiography or percutaneous coronary intervention: meta-analysis of individual patient data from 7 multicenter randomized clinical trials. Circulation. 2022;146(18):1329–43. https://doi.org/10.1161/CIRCULATIONAHA.122.061527.
Hammami R et al. Prevention of radial artery occlusion with rivaroxaban after transradial coronary procedures (RIVARAD). Presented at TCT 2022, Boston, MA, 2022.
Aminian A, Sgueglia GA, Wiemer M, et al. Distal versus conventional radial access for coronary angiography and intervention: the DISCO RADIAL trial. JACC Cardiovasc Interv. 2022;15(12):1191–201. https://doi.org/10.1016/j.jcin.2022.04.032.
Jolly SS, AlRashidi S, d’Entremont MA, et al. Routine ultrasonography guidance for femoral vascular access for cardiac procedures: the UNIVERSAL randomized clinical trial. JAMA Cardiol. 2022;7(11):1110–8. https://doi.org/10.1001/jamacardio.2022.3399.
Momin A. Total arterial revascularization gives the best long-term survival over 20 years. Presented at: STS 2022. January 29, 2022. Ottowa, Canada.
Saadat S. Multi-arterial coronary artery bypass grafting practice patterns in the USA: a report from the STS database. Presented at: STS 2022. January 29, 2022. Ottowa, Canada.
Sharma T, Krishnan AM, Lahoud R, Polomsky M, Dauerman HL. National trends in TAVR and SAVR for patients with severe isolated aortic stenosis. J Am Coll Cardiol. 2022;80(21):2054–6. https://doi.org/10.1016/j.jacc.2022.08.787.
Généreux P. The importance of pre-emptive TAVR in the future management of aortic stenosis. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Garcia S, Bapat V, Depta JP, et al. Frequency and safety of bioprosthetic valve fracture in patients undergoing valve-in-valve TAVR for failed surgical valves using SAPIEN 3/Ultra valves: insights from real-world data. Presented at: TCT 2022. Chicago, IL.
Tamm AR. JenaValve trilogy for treatment of aortic regurgitation: worldwide first results. Presented at: EuroPCR 2022. 2022; Paris, France.
Park DW, Ahn JM, Kang DY, et al. Edoxaban versus dual antiplatelet therapy for leaflet thrombosis and cerebral thromboembolism after TAVR: the ADAPT-TAVR randomized clinical trial. Circulation. 2022;146(6):466–79. https://doi.org/10.1161/CIRCULATIONAHA.122.059512.
Giustino G. Predictors of mortality after successful transcatheter aortic valve replacement: from the GALILEO trial. Presented at: EuroPCR 2022. 2022. Paris, France.
Kaur A. Efficacy and safety outcomes of cerebral embolic protection devices during transcatheter aortic valve replacement: an updated meta-analysis. Presented at: TVT 2022. 2022. Chicago, IL
Kapadia SR, Makkar R, Leon M, et al. Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. 2022;387(14):1253–63. https://doi.org/10.1056/NEJMoa2204961.
Tang G, Mahoney P, von Bardeleben RS, et al. One-year outcomes in patients with secondary MR outside the COAPT criteria: the MitraClip Global Expand study. Presented at: TVT 2022. 2022. Chicago, IL.
Lim DS, Smith RL, Gillam LD, et al. Randomized comparison of transcatheter edge-to-edge repair for degenerative mitral regurgitation in prohibitive surgical risk patients. JACC Cardiovasc Interv. 2022;15(24):2523–36. https://doi.org/10.1016/j.jcin.2022.09.005.
Zahid S, Ullah W, Hashem AM, et al. Transcatheter valve-in-valve implantation versus redo surgical mitral valve replacement in patients with failed mitral bioprostheses. EuroIntervention. 2022;18(10):824–35. https://doi.org/10.4244/EIJ-D-22-00437.
Hahn R. Transcatheter tricuspid valve repair: CLASP TR study one-year results. Presented at: EuroPCR 2022. 2022. Paris, France.
Baldus S. 30-day outcomes for transcatheter tricuspid repair: TriCLASP post-market study. Presented at: EuroPCR 2022. 2022. Paris, France.
Lurz P. Real-world outcomes for tricuspid edge-to-edge repair: initial 30-day results from the TriClip bRIGHT study. Presented at: EuroPCR 2022. 2022. Paris, France.
Sommer RJ, Aboulhosn JA. New SCAI guidelines: trying to close the holes in the PFO literature. JSCAI. 2022;1 (4):100039. https://doi.org/10.1016/j.jscai.2022.100039
Lakkireddy D. 3-year outcomes from the Amplatzer Amulet left atrial appendage occluder randomized controlled trial (Amulet IDE). Presented at: TCT 2022. September 17, 2022. Boston, MA.
Price MJ, Friedman DJ, Du C, et al. Comparative safety of transcatheter LAAO with the first-generation watchman and next-generation watchman FLX devices. JACC Cardiovasc Interv. 2022;15(21):2115–23. https://doi.org/10.1016/j.jcin.2022.09.002.
Freeman J, Higgins A, Wang Y, et al. Antithrombotic therapy after left atrial appendage occlusion in patients with atrial fibrillation. J Am Coll Cardiol. 2022;79(18):1785–98. https://doi.org/10.1016/j.jacc.2022.02.047.
Hochman JS, Anthopolos R, Reynolds HR, et al. Survival after invasive or conservative management of stable coronary disease. Circulation. 2023;147(1):8–19. https://doi.org/10.1161/CIRCULATIONAHA.122.062714.
McCune C, McKavanagh P, Menown IB. A review of the key clinical trials of 2015: results and implications. Cardiol Ther. 2016;5(2):109–132. https://doi.org/10.1007/s40119-016-0063-5. Epub 2016 Jun 8. PMID: 27277596; PMCID: PMC5125106
Douglas PS. Comparison of a precision care strategy with usual testing to guide management of stable patients with suspected coronary artery disease. Presented at: AHA 2022. November 6, 2022. Chicago, IL.
Batra G, Lindbäck J, Becker RC, et al. Biomarker-based prediction of recurrent ischemic events in patients with acute coronary syndromes. J Am Coll Cardiol. 2022;80:1735–47.
Shinke T. Impact of underlying causes of acute coronary syndrome on 1-year outcomes after percutaneous coronary intervention. Presented at: TCT 2022. September 19, 2022. Boston, MA.
Vranckx P. Management of high bleeding risk patients post bioresorbable polymer coated stent implantation with an abbreviated versus prolonged DAPT regimen. Presented at: EuroPCR 2022. May 17, 2022. Paris, France.
Bhatt DL. Bentracimab immediately and significantly reverses the antiplatelet effects of ticagrelor in older people. Presented at: ACC 2022. April 2, 2022. Washington, DC.
Aggarwal D, Bhatia K, Chunawala ZS, et al. P2Y12 inhibitor versus aspirin monotherapy for secondary prevention of cardiovascular events: meta-analysis of randomized trials. Eur Heart J Open. 2022;2(2):oeac019. Published 2022. https://doi.org/10.1093/ehjopen/oeac019
Wu H, Xu L, Zhao X, et al. Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation (OPTION): a randomized, open-label, endpoint-blinded. Noninferiority Trial Circ. 2023;147(3):212–22. https://doi.org/10.1161/CIRCULATIONAHA.122.062762.
Rao SV, Kirsch B, Bhatt DL, et al. A Multicenter, phase 2, randomized, placebo-controlled, double-blind, parallel-group, dose-finding trial of the oral factor xia inhibitor asundexian to prevent adverse cardiovascular outcomes after acute myocardial infarction [published correction appears in circulation. 2022 Dec 6;146(23):e330]. Circulation. 2022;146(16):1196–1206. https://doi.org/10.1161/CIRCULATIONAHA.122.061612
Shoamanesh A, Mundl H, Smith EE, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet. 2022;400(10357):997–1007. https://doi.org/10.1016/S0140-6736(22)01588-4.
Li Y, Liang Z, Qin L, et al. Bivalirudin plus a high-dose infusion versus heparin monotherapy in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: a randomised trial. Lancet. 2022;400(10366):1847–57. https://doi.org/10.1016/S0140-6736(22)01999-7.
Yang Y. The impact of Chinese herbal medicine tongxinluo in patients with acute myocardial infarction: results from the CTS-AMI trial. Presented at AHA 2022. November 6, 2022. Chicago, IL.
Wang Y, Zhu H, Hou X, et al. Randomized trial of left bundle branch vs. biventricular pacing for cardiac resynchronization therapy. J Am Coll Cardiol. 2022;80(13):1205–16. https://doi.org/10.1016/j.jacc.2022.07.019.
Vijayaraman P, Zalavadia D, Haseeb A, et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm. 2022;19(8):1263–71. https://doi.org/10.1016/j.hrthm.2022.04.023.
Vijayaraman P, Herweg B, Verma A, et al. Rescue left bundle branch area pacing in coronary venous lead failure or nonresponse to biventricular pacing: results from International LBBAP Collaborative Study Group. Heart Rhythm. 2022;19(8):1272–80. https://doi.org/10.1016/j.hrthm.2022.04.024.
Jastrzębski M, Kiełbasa G, Cano O, et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J. 2022;43(40):4161–73. https://doi.org/10.1093/eurheartj/ehac445. (PMID: 35979843).
Carina Blomström-Lundqvist, Vassil Traykov, Paola Anna Erba et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections—endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID), and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2020; 41(21):2012–2032
Khubrani R, Alghamdi AS, Alsubaie AA, Alenazi T, Almutairi A, Alsunaydi F. Rate of cardiovascular implantable electronic device-related infection at a tertiary hospital in saudi arabia: a retrospective cohort study. Cureus. 2022;14(7):e27078. Published 2022. https://doi.org/10.7759/cureus.27078
Knops RE, Pepplinkhuizen S, Delnoy PPHM et al. Device-related complications in the subcutaneous and transvenous ICD: a secondary analysis of the PRAETORIAN trial. Eur Heart J. 2022.
Gold MR, Lambiase PD, El-Chami MF, et al. Primary results from the understanding outcomes With the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation. 2021;143(1):7–17.
Healey J. The ATLAS trial: avoid transvenous leads in appropriate subjects. Presented at: HRS 2022. April 30, 2022. San Francisco
Hadwiger M, Dagres N, Haug J, et al. Survival of patients undergoing cardiac resynchronization therapy with or without defibrillator: the RESET-CRT project. Eur Heart J. 2022;43(27):2591–9. https://doi.org/10.1093/eurheartj/ehac053.
Aktas MK. The benefit of an implantable cardioverter-defibrillator in heart failure patients treated with empagliflozin: an analysis from the EMPEROR-Reduced trial. Presented at: HRS 2022. 2022; San Francisco, CA.
Samuel M, et al. Reduction in shock burden with catheter ablation versus escalated antiarrhythmic drug therapy: insights from the VANISH trial. News from ventricular ablation, EHRA 2022, 3–5 April, Copenhagen, Denmark
Dagres N, et al. Cardiac magnetic resonance imaging for prediction of risk for sudden cardiac death after myocardial infarction, the updated PROFID clinical prediction model. Late-breaking science 1, EHRA 2022, 3–5 April, Copenhagen, Denmark
Jons C. BIO|GUARD-MI: biomonitoring in patients with preserved left ventricular function after diagnosed myocardial infarction. Presented at: ACC 2022. April 4, 2022.
Guo Y, Zhang H, Lip GYH; mAF-App II Trial investigators. Consumer-led screening for atrial fibrillation: a report from the mAFA-II trial long-term extension cohort. JACC Asia. 2022;2(6):737–746. Published 2022 Nov 1. https://doi.org/10.1016/j.jacasi.2022.07.006
Rizas KD, Freyer L, Sappler N, et al. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med. 2022;28(9):1823–30. https://doi.org/10.1038/s41591-022-01979-w.
Zeitler EP, Bunch TJ, Khanna R, Fan X, Iglesias M, Russo AM. Comparative risk of dementia among patients with atrial fibrillation treated with catheter ablation versus anti-arrhythmic drugs. Am Heart J. 2022;254:194–202. https://doi.org/10.1016/j.ahj.2022.09.007.
Harskamp RE, Fanaroff AC, Lopes RD, et al. Antithrombotic therapy in patients with atrial fibrillation after acute coronary syndromes or percutaneous coronary intervention. J Am Coll Cardiol. 2022;79:417–27.
Connolly SJ, Karthikeyan G, Ntsekhe M, et al., on behalf of the INVICTUS Investigators. Rivaroxaban in Rheumatic Heart Disease–Associated Atrial Fibrillation. N Engl J Med 2022;387:978–88.
Castrejón-Castrejón S, Martínez Cossiani M, Jáuregui-Abularach M, et al. Multicenter prospective comparison of conventional and high-power short duration radiofrequency application for pulmonary vein isolation: the high-power short-duration radiofrequency application for faster and safer pulmonary vein ablation (POWER FAST III) trial [published online ahead of print, 2023 Feb 18]. J Interv Card Electrophysiol. 2023. https://doi.org/10.1007/s10840-023-01509-9
Lemoine M, et al. Pulmonary vein isolation by pulsed-field ablation induces smaller neurocardiac damage than cryoballoon ablation. Atrial fibrillation and ablation, EHRA 2022, 3–5 April, Copenhagen, Denmark
Kueffer T, et al. Pulsed field ablation of atrial fibrillation: Recurrence rate after first pulmonary vein isolation and first insights into durability and redo procedures. Poster Session, EHRA 2022, 3–5 April.
Andrade JG, Deyell MW, Macle L, et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med. 2023;388(2):105–16. https://doi.org/10.1056/NEJMoa2212540.
Piccini JP. Efficacy and safety of botulinum toxin type A for the prevention of postoperative atrial fibrillation in cardiac surgery patients: results from the phase 2 NOVA study. Presented at: AHA 2022. 2022; Chicago
Stambler BS, Plat F, Sager PT, et al. First randomized, multicenter, placebo-controlled study of self-administered intranasal etripamil for acute conversion of spontaneous paroxysmal supraventricular tachycardia (NODE-301). Circ Arrhythm Electrophysiol. 2022;15(12):e010915. https://doi.org/10.1161/CIRCEP.122.010915
Kearney A, Linden K, Savage P, Menown IBA. Advances in clinical cardiology 2020: a summary of key clinical trials. Adv Ther. 2021;38(5):2170–200. https://doi.org/10.1007/s12325-021-01711-z.
Lewis GD, Voors AA, Cohen-Solal A, et al. Effect of Omecamtiv Mecarbil on exercise capacity in chronic heart failure with reduced ejection fraction. JAMA. 2022;328(3):259–69. https://doi.org/10.1001/jama.2022.11016.
Butler J, Anker SD, Lund LH, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: the DIAMOND trial. Eur Heart J. 2022;43(41):4362–73. https://doi.org/10.1093/eurheartj/ehac401.
Ghazi L, Yamamoto Y, Riello R, et al. Electronic alerts to improve heart failure therapy in outpatient practice. J Am Coll Cardiol. 2022;79(22):2203–13. https://doi.org/10.1016/j.jacc.2022.03.338.
Castellano JM, Pocock SJ, Bhatt DL, et al. Polypill strategy in secondary cardiovascular prevention. N Engl J Med. 2022;26(387):967–77. https://doi.org/10.1056/NEJMoa2208275.
Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists' Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788–1801. https://doi.org/10.1016/S0140-6736(22)02074-8
EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2022. https://doi.org/10.1056/NEJMoa2204233
Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multinational randomized trial. Nat Med. 2022;28:568–74. https://doi.org/10.1038/s41591-021-01659-1.
Solomon SD, McMurray JJV, Claggett B, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022;27(387):1089–98. https://doi.org/10.1056/NEJMoa2206286.
Mullens W, Dauw J, Martens P, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med. 2022;26(387):1185–95. https://doi.org/10.1056/NEJMoa2203094.
Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. The Lancet. 2022;400(10367):1938–52. https://doi.org/10.1016/S0140-6736(22)02076-1.
Kalra PR, Cleland JGF, Petrie MC et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): an investigator-initiated, prospective, randomised, open-label, blinded-endpoint trial. The Lancet. 2022. https://doi.org/10.1016/S0140-6736(22)02083–9.
Desai MY, Owens A, Geske JB, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol. 2022;80(2):95–108. https://doi.org/10.1016/j.jacc.2022.04.048.
O’Donoghue ML, Rosenson RS, Gencer B, et al. Small interfering RNA to reduce lipoprotein(a) in cardiovascular disease. N Engl J Med. 2022;387(20):1855–64. https://doi.org/10.1056/NEJMoa2211023.
Rider DA, Eisermann M, Löffler K, et al. Pre-clinical assessment of SLN360, a novel siRNA targeting LPA, developed to address elevated lipoprotein (a) in cardiovascular disease. Atherosclerosis. 2022;349:240–7. https://doi.org/10.1016/j.atherosclerosis.2022.03.029.
Collaboration CTT. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. The Lancet. 2022;400(10355):832–45. https://doi.org/10.1016/S0140-6736(22)01545-8.
O’Donoghue ML, Giugliano RP, Wiviott SD et al. Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. Circulation. 2022; 11;146(15):1109–19. https://doi.org/10.1161/CIRCULATIONAHA.122.061620
Mackenzie IS, Hawkey CJ, Ford I, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. The Lancet. 2022;400(10359):1195–205. https://doi.org/10.1016/S0140-6736(22)01657-9.
Schlaich MP, Bellet M, Weber MA, et al. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. The Lancet. 2022;400(10367):1927–37. https://doi.org/10.1016/S0140-6736(22)02034-7.
Sun Y, Mu J, Wang DW, et al. A village doctor-led multifaceted intervention for blood pressure control in rural China: an open, cluster randomised trial. Lancet. 2022;399(10339):1964–75. https://doi.org/10.1016/S0140-6736(22)00325-7.
Ho CLB, Chowdhury EK, Doust J, et al. The effect of taking blood pressure lowering medication at night on cardiovascular disease risk. A systematic review. J Hum Hypertens. 2021;35:308–14.
Mackenzie IS, Rogers A, Poulter NR, et al., on behalf of the TIME study group. Cardiovascular outcomes in adults with hypertension with evening versus morning dosing of usual antihypertensives in the UK (TIME Study): a prospective, randomized, open-label, blinded-endpoint clinical trial. Lancet 2022;400:1417–25.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Author Contributions
PS—Lead author, collated clinical trials for review and divided sections across authors. Wrote structural, ACS and antiplatelet sections as well as abstract, introduction and summary table. Compiled all sections into one document and collated references. Implemented final edits from supervising consultant and reviewers. BC—Wrote intervention section. JF—Wrote Heart failure and prevention section; MS—Wrote EP/Devices section; IM—Supervising consultant. Guided style and format of review paper. Reviewed and edited final document with changes fed back to and incorporated into final paper by lead author.
Disclosures
Ian Menown has received grants to institution, honoraria and/or conference sponsorship from Biosensors, Meril Life, and Bayer. Other authors have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Savage, P., Cox, B., Shahmohammadi, M. et al. Advances in Clinical Cardiology 2022: A Summary of Key Clinical Trials. Adv Ther 40, 2595–2625 (2023). https://doi.org/10.1007/s12325-023-02502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02502-4