Abstract
Introduction
Direct oral anticoagulants (DOACs) are essential in ischemic stroke/systemic embolism (SE) prevention among patients with nonvalvular atrial fibrillation (NVAF). This study compared the risk of ischemic stroke/SE among patients with NVAF who discontinued DOACs following the first fill (“one-and-done”) relative to patients who continued DOACs beyond the first fill (“continuers”).
Methods
De-identified data from Symphony Health, an ICON plc Company, PatientSource®, April 1, 2017 to October 31, 2020, were used to identify adults with NVAF initiated on DOACs (index date). Patients with only one DOAC claim during the 90-day landmark period starting on the index date were classified as one-and-done and the remaining as continuers. Inverse probability of treatment weighting was used to balance baseline characteristics in the cohorts. Time from the landmark period end to the first ischemic stroke/SE event or, among those without the event, to clinical activity or data end was compared between balanced cohorts using survival analysis.
Results
Of patients initiating DOACs, 23.6% were classified as one-and-done users. After weighting was performed, 241,159 and 238,889 patients comprised the one-and-done and continuer cohorts, respectively. At 12 months of follow-up, the probability of ischemic stroke/SE was 1.44% in the one-and-done cohort and 1.00% in the continuer cohort [hazard ratio (95% confidence interval) 1.44 (1.34–1.54); p < 0.0001]. Results at earlier and later time points and in a sensitivity analysis with a 75-day landmark period were similar.
Conclusion
A substantial proportion of patients were one-and-done DOAC users, which was associated with significantly higher risk of ischemic stroke/SE events. There is an unmet need to improve access and encourage continuous use of DOACs among patients with NVAF so that severe and fatal complications may be mitigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Long-term and compliant anticoagulation therapy is critical for the prevention of thromboembolic complications in atrial fibrillation (AF), since it significantly reduces the risk of ischemic stroke and in-hospital mortality. |
There is scarce literature evaluating the clinical consequences of early direct oral anticoagulant (DOAC) discontinuation; therefore, this study was conducted to compare the risk of ischemic stroke and systemic embolism (SE) among patients with nonvalvular atrial fibrillation (NVAF) who discontinued DOAC therapy following the first fill (“one-and-done”) compared to patients who continued DOACs beyond the first fill (“continuers”). |
What was learned from the study? |
In this study, 23.6% of patients with NVAF initiating DOACs were classified as one-and-done users. |
At 12 months of follow up, the risk of ischemic stroke or SE as a composite outcome was 44% higher among one-and-done DOAC users compared to DOAC continuers; the magnitude of this risk remained similar both short- and long-term. |
There is a need to better understand the factors associated with discontinuation of DOACs to inform policies aimed at maximizing the benefit of these agents among patients with NVAF. |
Introduction
Atrial fibrillation (AF) is a form of cardiac arrhythmia characterized by abnormal and uncoordinated contractions of the heart’s atria [1]. The prevalence of AF has increased over the last 5 decades and is continuing to increase globally [2]. In the USA, the age-standardized prevalence rate of AF was 900 per 100,000 people in 2017 [3], currently affecting at least 3–6 million people [2].
Severe and often fatal complications, such as ischemic stroke and systemic embolism (SE), are common consequences of AF [1, 4]. Notably, patients with AF have an approximately fivefold higher risk of ischemic stroke and eightfold higher risk of having multiple cardiovascular hospitalizations compared to those without AF [5, 6]. Additionally, strokes related to AF tend to be more severe than those with other underlying causes [5], resulting in higher rates of adverse events and mortality [7], as well as higher hospitalization costs [8].
As such, long-term anticoagulation therapy is critical for the prevention of thromboembolic complications in AF [1]. Indeed, therapeutic anticoagulation has been shown to reduce the odds of moderate or severe ischemic stroke and in-hospital mortality among patients with AF in real-world clinical practice [9]. In patients with nonvalvular AF (NVAF), direct oral anticoagulants (DOACs) are recommended over warfarin on the basis of the results of four clinical trials comparing dabigatran, rivaroxaban, apixaban, or edoxaban (i.e., the four DOACs that are currently approved by the US Food and Drug Administration for NVAF [10,11,12,13]) against warfarin [14]. In a network meta-analysis of these four trials, standard-dose DOACs were associated with a significantly lower risk of stroke or SE (hazard ratio [HR; 95% confidence interval (CI)] = 0.81 [0.74, 0.89]), death (0.92 [0.87, 0.97]), and intracranial bleeding (0.45 [0.37, 0.56]) compared to warfarin [15]. Therefore, guidelines recommend the use of DOACs as first-line anticoagulation, with periodic reevaluation to assess the need for further anticoagulant therapy [14]. In the USA, the cost of DOACs may be partially covered by public (i.e., Medicare, Medicaid) or private/commercial insurance, while a portion is paid out-of-pocket by the patient.
Despite the proven efficacy of DOACs, patient compliance remains a challenge [16, 17]. Prior real-world studies have reported discontinuation rates ranging from 17.9% to 27.4% at 3 months to 4.5–14.9% at 2 years of follow-up [18, 19]. Additionally, nearly 40% of DOAC discontinuations occurred within the first 4 months in one international registry-based study [20]. Importantly, discontinuation of DOACs after a median 182 days of treatment was associated with increased risk of ischemic stroke/SE among patients with AF [20]. Given the large proportion of discontinuations occurring in the first few months of DOAC initiation, there is a need to evaluate the clinical consequences associated with these shorter durations of treatment; however, there is scarce literature doing so. Therefore, the current study was conducted to describe and compare the risk of ischemic stroke and SE among patients with NVAF who discontinued DOAC therapy following the first fill (i.e., up to 1 month of therapy) compared to patients who continued DOACs beyond the first fill.
Methods
Data Source
De-identified data from Symphony Health, an ICON plc Company, PatientSource®, April 1, 2017 to October 31, 2020, were used. This open claims data source contains patient demographics, medical and procedure claims, and prescription drug claims, including the status of prescription drug claims (i.e., approved, rejected, abandoned). The open claims nature means that a patient’s healthcare activity is captured regardless of maintaining the same healthcare plan if the patient uses providers from the network that supplies data to the database. The database captures more than 75% of all US retail prescription claims, representing over three-quarters of the US population annually across multiple payer channels (i.e., commercial, Medicare, Medicaid); the remaining less than 25% of claims are outside of the covered networks.
Study Design
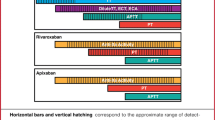
The study had a retrospective longitudinal design (Fig. 1). The index date was the date of the first DOAC claim (i.e., apixaban, dabigatran, or rivaroxaban). The study focused on these three DOACs as the most commonly used in the USA [21]. Only patients with the status of the first claim being “approved” (i.e., submitted by a pharmacy and approved for payment by health plans after claims adjudication) were retained.
The baseline period was defined as the 6-month period of clinical activity before the index date, where clinical activity was based on the first and last patient-level activity flags (i.e., either a pharmacy or a medical claim).
The 90-day landmark period starting on the index date was used to classify patients into the “one-and-done” and “continuer” cohorts. The duration of the landmark period was based on a 60-day or longer gap in DOAC supply to define DOAC discontinuation after 30 or fewer days of the index DOAC claim supply. The gap of 60 days or longer was chosen since it has been commonly used in prior literature to define DOAC discontinuation in NVAF [22, 23], and patients with more than 30 days of supply for the index DOAC claim were excluded since it would not have been possible to classify them as “one-and-done” or “continuer” during the 90-day landmark period (see “Sample Selection”). A sensitivity analysis was conducted using a gap of at least 45 days to define discontinuation, reducing the landmark period length and associated survival bias (see “Sensitivity Analysis”).
The follow-up period used to measure the outcomes started on day 91 post-index and continued until the earliest of the end of clinical activity or data availability.
Sample Selection
Patients with the first claim for apixaban, dabigatran, or rivaroxaban were included in the study if they met the following inclusion criteria: (1) at least 6 months of clinical activity before the index date; (2) no claims for other oral anticoagulants (i.e., betrixaban, edoxaban, warfarin) before the index date; (4) at least one claim with a diagnosis for AF (International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] code I48) during the baseline period or on the index date; and (5) at least 18 years old on the index date.
Patients were excluded from the study if they had (1) at least one claim with any of the following diagnoses or procedures at baseline: mitral stenosis, mechanical heart-valve, venous thromboembolism (VTE), hip or knee replacement surgery, or organ or tissue replaced by transplant; (2) pregnancy during or after the baseline period; (3) at least one claim with a diagnosis of stroke (ischemic or hemorrhagic) or SE during an inpatient admission on the index date or within 30 days prior to the index date (to avoid confounding with the outcomes of ischemic stroke and SE); (4) more than one claim for DOACs (i.e., apixaban, betrixaban, dabigatran, edoxaban, rivaroxaban) on the index date; or (5) more than one final claim status (e.g., approved and abandoned) for the index DOAC on the index date.
Additionally, retaining only patients with the “approved” status of the first claim, the following exclusion criteria for the identification of cohorts during the landmark period were applied: (1) 90 or fewer days of clinical activity post-index (i.e., incomplete landmark period); (2) stroke or SE diagnosis in an inpatient setting during the landmark period; and (3) more than 30 days of supply on the index DOAC claim.
During the landmark period, patients were classified into the “one-and-done” cohort if they discontinued DOAC therapy after the first DOAC claim, i.e., did not have another approved DOAC claim during the landmark period (a 60-day or longer gap in DOAC supply). Patients were classified into the “one-and-done” cohort if they persisted on DOAC therapy beyond the first claim, i.e., had at least two approved DOAC claims during the landmark period.
Outcome Measures
The first instance of ischemic stroke (ICD-10-CM code I63) and SE (ICD-10-CM code I74) were measured in each cohort during the follow-up period as a composite outcome (i.e., ischemic stroke/SE) and separately. Only diagnoses in claims from an inpatient setting were considered.
Statistical Analysis
To balance baseline characteristics between the one-and-done and continuer cohorts, inverse probability of treatment weighting (IPTW) was applied. The propensity score was computed from a logistic regression model and adjusted for demographic characteristics (i.e., age, sex, region of residence, insurance plan type, index year); comorbidities (Quan–Charlson Comorbidity Index [Quan-CCI] [24], CHA2DS2-VASc score, HAS-BLED score); recent ischemic or hemorrhagic stroke/SE (i.e., presence of event and time from the event to index date); polypharmacy (i.e., use of at least five different medications concurrently); use of antihypertensive agents, antihyperlipidemic agents, antiplatelet agents; the index DOAC medication; and all-cause pharmacy costs. The balance of baseline characteristics was assessed using standardized differences (less than 10% indicated balance) [25].
The probability of an ischemic stroke or SE event during the follow-up period was described between the one-and-done cohort and the continuer cohort using weighted Kaplan–Meier survival analysis. The risk of an ischemic stroke or SE event was compared between the two cohorts using weighted Cox proportional hazard models, with HRs and their 95% CIs and p values reported. Time to the ischemic stroke or SE event was defined as the time from day 91 post-index (i.e., first day following the landmark period) until the first ischemic stroke or SE event during the follow-up period; patients for whom the event was not observed during the follow-up period were censored at the end of the follow-up period.
Sensitivity Analysis
A sensitivity analysis using a 75-day landmark period was conducted to minimize the loss of patients with early ischemic stroke and SE events (i.e., within the duration of the landmark period).
Compliance with Ethics Guidelines
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) [26].
Results
Among 779,468 patients prescribed with a DOAC who had NVAF and met other inclusion and exclusion criteria, 667,417 (85.6%) had their first DOAC claim approved. Furthermore, 480,048 patients met additional criteria during the landmark period, and among them, 113,336 (23.6%) were classified into the one-and-done cohort and 366,712 (76.4%) into the continuer cohort (Fig. 2). Baseline characteristics of the unweighted cohorts are described in Table S1 in the supplementary material.
Identification of NVAF population. AF atrial fibrillation, DOAC direct oral anticoagulant, NVAF non-valvular atrial fibrillation. *Patients were excluded from the study population if they had (1) ≥ 1 claim with any of the following diagnoses or procedures at baseline: mitral stenosis, mechanical heart-valve, venous thromboembolism, hip or knee replacement surgery, organ or tissue replaced by transplant; (2) pregnancy during or after the baseline period; (3) ≥ 1 claim with a diagnosis of stroke (ischemic or hemorrhagic) or systemic embolism during an inpatient admission on the index date or within 30 days prior to the index date; (4) > 1 claim for DOACs (i.e., apixaban, betrixaban, dabigatran, edoxaban, rivaroxaban) on the index date; or (5) > 1 final claim status (e.g., approved and abandoned) for the index DOAC on the index date. †Patients were excluded during the landmark period if they had (1) ≤ 90 days of clinical activity post-index; (2) stroke or systemic embolism diagnosis in an inpatient setting within the first 90 days post-index (i.e., during landmark period); or (3) > 30 days of supply on the index DOAC claim
Study Population and Weighted Baseline Characteristics
After the two cohorts were weighted to balance baseline characteristics, a total of 241,159 patients were included in the one-and-done cohort and 238,889 patients in the continuer cohort. On the basis of the standardized differences, the weighted cohorts were well balanced (Table 1). Mean [standard deviation (SD)] age was 69.1 [10.1] years in the one-and-done cohort and 69.2 [9.5] years in the continuer cohort, and 45.1% of patients were female in both cohorts. Most patients were covered by Medicare (55.0% in the one-and-done cohort and 54.2% in the continuer cohort), followed by commercial insurance (36.2% and 37.2%), Medicaid (7.6% and 7.5%), and another type of insurance (1.3% and 1.2%). The mean [SD] patient out-of-pocket paid amount for the index drug claim was $77.90 [$157.50] in the one-and-done cohort and $67.60 [$137.70] in the continuer cohort. The mean [SD] CHA2DS2-VASc score was 3.0 [1.6] among patients in the one-and-done cohort and 3.0 [1.7] among patients in the continuer cohort. In both cohorts, 1.0% and 0.1% of patients had a baseline inpatient diagnosis of ischemic stroke and SE, respectively. Patients in the one-and-done and continuer cohorts had similar prevalence of risk factors for stroke, with the most common being hypertension (67.0% and 67.8%, respectively), hyperlipidemia (45.8% and 45.4%), diabetes (27.1% and 26.4%), and congestive heart failure (26.5% and 26.7%).
Probability of Ischemic Stroke and SE
The mean [SD] duration of follow-up was 15.0 [9.5] months in the one-and-done cohort and 15.7 [9.4] months in the continuer cohort. At 3 months of follow-up, the probability of ischemic stroke or SE as a composite outcome was 0.41% in the one-and-done cohort compared to 0.28% in the continuer cohort (log-rank p < 0.0001; Fig. 3). This higher probability of ischemic stroke or SE event in the one-and-done compared to continuer cohort remained consistent at later points of follow-up, with probabilities of 1.44% in the one-and-done cohort and 1.00% in the continuer cohort at 12 months (log-rank p < 0.0001).
Ischemic stroke or SE probability in weighted one-and-done versus continuer cohorts. CI confidence interval, DOAC direct oral anticoagulant, SE systemic embolism. Ischemic stroke and SE events were identified in an inpatient setting. The time to the first event was measured from day 91 post-index date (i.e., first day after the end of the landmark period); patients for whom the event was not observed during the follow-up period were censored at the end of the follow-up period. †p < 0.05
The probability of the composite endpoint was mainly driven by that of ischemic stroke. At 3 months of follow-up, the probability of ischemic stroke alone was 0.38% in the one-and-done cohort compared to 0.26% in the continuer cohort, while at 12 months, these probabilities were 1.33% and 0.92%, respectively (all log-rank p < 0.0001; data not shown).
The risk of ischemic stroke or SE as a composite outcome was 45% higher in the one-and-done cohort than in the continuer cohort at 3 months, 42% higher at 6 months, 44% higher at 12 months, and 42% higher at 24 months of follow-up (all p < 0.0001; Fig. 4). Similar results were observed for ischemic stroke and SE alone (Fig. 4).
Sensitivity Analysis Using a 75-Day Landmark Period
When a 75-day landmark period was used, a total of 245,433 patients comprised the weighted one-and-done cohort and 243,488 patients comprised the weighted continuer cohort. The probabilities of ischemic stroke or SE in the two cohorts were comparable to those of the main analysis (Fig. 5). The risk of ischemic stroke or SE as a composite outcome was 43–48% higher in the one-and-done cohort than the continuer cohort at 3, 6, 12, and 24 months of follow-up (all p < 0.0001; Fig. 6).
Ischemic stroke or SE probability in weighted one-and-done versus continuer cohorts (75-day landmark period). CI confidence interval, DOAC direct oral anticoagulant, SE systemic embolism. Ischemic stroke and SE events were identified in an inpatient setting. The time to the first event was measured from day 76 post-index date (i.e., first day after the end of the landmark period); patients for whom the event was not observed during the follow-up period were censored at the end of the follow-up period. †p < 0.05
Risk of ischemic stroke or SE in weighted one-and-done versus continuer cohorts (75-day landmark period). HR hazard ratio, CI confidence interval, SE systemic embolism. Ischemic stroke and SE were identified in an inpatient setting. HRs were generated using univariate weighted Cox proportional hazard models. †p < 0.05
Discussion
In this retrospective real-world study of patients with NVAF, 23.6% discontinued DOAC treatment for at least 60 days after the first prescription fill (i.e., after up to 1 month of therapy) and 76.4% persisted on DOAC therapy after the first fill. It is important to note that the proportion of patients with one-and-done status is an approximate estimate, as about 30% of the patients initiating DOAC therapy were impossible to classify as either one-and-done or continuers on the basis of retrospective data. Discontinuation of DOACs after up to a month of therapy was associated with a significantly higher risk of ischemic stroke or SE, with the magnitude of this increased risk being similar both short-term and long-term. Thus, while it is possible that some patients who initially discontinued DOACs restarted the therapy later, the findings of our study suggest that the association of the initial discontinuation with negative patient outcomes does not weaken over time. These results remained robust with a shorter landmark period in the sensitivity analysis.
Treatment guidelines do not specify an optimal duration of anticoagulation among patients with AF, instead recommending reevaluation at periodic intervals to reassess stroke risk and if there is a continued need for anticoagulation [14]. Although our study did not evaluate reasons for DOAC discontinuation, it is possible that the lack of specific guideline recommendations may have contributed to decisions to stop therapy in clinical practice. Additionally, the risk or occurrence of bleeding or patient out-of-pocket costs may also be obstacles to continuous DOAC therapy [16, 27]. For instance, Rome et al. found that commercially insured patients with AF who had high DOAC copayments had higher rates of discontinuation than those with low copayments [27]. In our study, the proportions of patients with major bleeding at baseline and baseline HAS-BLED score were balanced between the one-and-done and continuer cohorts; patient out-of-pocket paid amounts for the index drug claim were also similar. Further study is warranted to confirm which reasons may prompt patients with NVAF to discontinue DOAC treatment after the first prescription fill.
This study is the first to evaluate the association between one-and-done DOAC use status and patient outcomes in NVAF, filling the prior knowledge gap regarding early DOAC discontinuation and its related clinical consequences. While previous literature has focused on discontinuation following longer durations of DOAC treatment, it is largely consistent with our findings on the negative consequences of DOAC noncompliance. For instance, in the GARFIELD-AF registry study, after the median time on treatment of 182 days, discontinuation of DOACs for at least 7 days was associated with more than twofold increase in the risk of ischemic stroke/SE (HR [95% CI] = 2.21 [1.42, 3.44]) [20]. Additionally, a meta-analysis of real-world AF studies found that DOAC nonpersistence was associated with increased risk of stroke/transient ischemic attack (pooled HR [95%] = 4.55 [2.80, 7.39]) [17]. The findings of the current study corroborate prior literature and contribute additional insight into the elevated risk of stroke/SE among patients who specifically discontinue DOAC treatment within the first month of therapy.
Ischemic stroke in patients with AF can result in serious clinical and economic consequences requiring emergency and specialized care [28]. Indeed, the healthcare resource utilization associated with stroke is high; in 2018, there were 904,000 inpatient discharges and 802,000 emergency department visits with stroke as the principal diagnosis [29]. Moreover, survivors of AF-related ischemic stroke are more likely to have longer hospital stays, disability, and long-term care than survivors of stroke from other causes [30]. This high healthcare use translates to a large economic burden [8, 29]. In one claims-based study of adult patients with ischemic stroke, the cost of a hospital admission with a primary diagnosis of ischemic stroke was $23,770 for patients with AF, which was $4991 higher than the cost for patients without AF [8]. Given the substantial clinical and economic burden associated with AF-related ischemic stroke, there is an urgent unmet need to ensure proper access and improve patient compliance to DOACs so that downstream risk of ischemic stroke/SE can be minimized. While anticoagulation clinics were traditionally used for monitoring and dose adjustment of warfarin, their expansion to additionally provide patient education and periodic follow-up for those receiving DOACs may help to promote ongoing treatment adherence [31]. Additional research is warranted to identify factors associated with early DOAC treatment discontinuation and how they impact subsequent clinical and economic outcomes, which may inform treatment policies and decision-making in real-world practice.
Limitations
Some limitations apply to the findings of this study. Any diagnoses, services, or prescriptions received from providers outside of the network were not captured. For instance, patients who switched to a different claims transaction network for their prescriptions may have appeared as less compliant. Additionally, drug samples were not captured in the data; however, if patients in the one-and-done cohort received free samples, this would have led to more conservative risk estimates. Furthermore, a survival bias may have been introduced as a result of the landmark period design (i.e., the requirement of at least 90 days post-index without stroke/SE events); particularly, patients with only one approved DOAC fill may have been less likely to survive beyond 90 days than patients who continued DOAC treatment. However, the sensitivity analysis with a shorter landmark period (i.e., 75 days) yielded comparable results. Finally, as with all claims-based analyses, this study may have been subject to residual confounding due to unmeasured confounders (i.e., information not available in transactions data).
Conclusions
This retrospective study demonstrated that close to a quarter of patients with NVAF discontinued DOACs after up to a month of therapy, and these patients had consistently higher risk of ischemic stroke or SE short- and long-term than patients who continued DOAC therapy. There is a need to better understand the factors associated with discontinuation of DOACs to inform policies aimed at maximizing the benefit of these agents among patients with NVAF. Future studies may also evaluate the economic impact of DOAC discontinuation after the first prescription fill.
References
Brundel B, Ai X, Hills MT, et al. Atrial fibrillation. Nat Rev Dis Primers. 2022;8(1):21. https://doi.org/10.1038/s41572-022-00347-9.
Kornej J, Borschel CS, Benjamin EJ, et al. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127(1):4–20. https://doi.org/10.1161/CIRCRESAHA.120.316340.
Dai H, Zhang Q, Much AA, et al. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the Global Burden of Disease Study 2017. Eur Heart J Qual Care Clin Outcomes. 2021;7(6):574–82. https://doi.org/10.1093/ehjqcco/qcaa061.
Chung MK, Refaat M, Shen WK, et al. Atrial fibrillation: JACC Council perspectives. J Am Coll Cardiol. 2020;75(14):1689–713. https://doi.org/10.1016/j.jacc.2020.02.025.
Centers for Disease Control and Prevention. Atrial fibrillation. https://www.cdc.gov/heartdisease/atrial_fibrillation.htm#print. Accessed 2022 Sept 11.
Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–20. https://doi.org/10.1161/CIRCOUTCOMES.110.958165.
Keller K, Hobohm L, Wenzel P, et al. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Heart Rhythm. 2020;17(3):383–90. https://doi.org/10.1016/j.hrthm.2019.10.001.
Wang G, Joo H, Tong X, et al. Hospital costs associated with atrial fibrillation for patients with ischemic stroke aged 18–64 years in the United States. Stroke. 2015;46(5):1314–20. https://doi.org/10.1161/STROKEAHA.114.008563.
Xian Y, O’Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in-hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317(10):1057–67. https://doi.org/10.1001/jama.2017.1371.
Boehringer Ingelheim Pharmaceuticals, Inc. Highlights of Prescribing Information PRADAXA® (dabigatran etexilate) 2021. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed 2022 Sept 9.
Bristol-Myers Squibb Company. Highlights of Prescribing Information ELIQUIS® (apixaban) 2021. https://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 2022 Sept 9.
Daiichi Sankyo Co., Ltd. Highlights of Prescribing Information SAVAYSA®(edoxaban) 2021. https://daiichisankyo.us/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed 2022 Sept 22.
Janssen Pharmaceuticals, Inc. Highlights of Prescribing Information XARELTO® (rivaroxaban) 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf. Accessed 2022 Sept 9.
January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125–51. https://doi.org/10.1161/CIR.0000000000000665.
Carnicelli AP, Hong H, Connolly SJ, et al. Direct oral anticoagulants versus warfarin in patients with atrial fibrillation: patient-level network meta-analyses of randomized clinical trials with interaction testing by age and sex. Circulation. 2022;145(4):242–55. https://doi.org/10.1161/CIRCULATIONAHA.121.056355.
Tarn DM, Shih KJ, Schwartz JB. Reasons for nonadherence to the direct oral anticoagulant apixaban for atrial fibrillation. J Am Geriatr Soc. 2021;69(12):3683–7. https://doi.org/10.1111/jgs.17423.
Ozaki AF, Choi AS, Le QT, et al. Real-world adherence and persistence to direct oral anticoagulants in patients with atrial fibrillation: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2020;13(3):e005969. https://doi.org/10.1161/CIRCOUTCOMES.119.005969.
Buck J, Fromings Hill J, Martin A, et al. Reasons for discontinuing oral anticoagulation therapy for atrial fibrillation: a systematic review. Age Ageing. 2021;50(4):1108–17. https://doi.org/10.1093/ageing/afab024.
Coleman CI, Tangirala M, Evers T. Treatment persistence and discontinuation with rivaroxaban, dabigatran, and warfarin for stroke prevention in patients with non-valvular atrial fibrillation in the United States. PLoS ONE. 2016;11(6):e0157769. https://doi.org/10.1371/journal.pone.0157769.
Cools F, Johnson D, Camm AJ, et al. Risks associated with discontinuation of oral anticoagulation in newly diagnosed patients with atrial fibrillation: results from the GARFIELD-AF Registry. J Thromb Haemost. 2021;19(9):2322–34. https://doi.org/10.1111/jth.15415.
Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35(8):2505–7. https://doi.org/10.1007/s11606-020-05904-0.
Berger JS, Laliberte F, Kharat A, et al. Real-world effectiveness and safety of rivaroxaban versus warfarin among non-valvular atrial fibrillation patients with obesity in a US population. Curr Med Res Opin. 2021;37(6):881–90. https://doi.org/10.1080/03007995.2021.1901223.
Laliberte F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30(7):1317–25. https://doi.org/10.1185/03007995.2014.907140.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. https://doi.org/10.1093/aje/kwq433.
Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–34. https://doi.org/10.1080/03610910902859574.
U.S. Department of Health and Human Services. 45 CFR 46: pre-2018 requirements. https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.101. Accessed 2020 Oct 16.
Rome BN, Gagne JJ, Avorn J, et al. Non-warfarin oral anticoagulant copayments and adherence in atrial fibrillation: a population-based cohort study. Am Heart J. 2021;233:109–21. https://doi.org/10.1016/j.ahj.2020.12.010.
Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nat Rev Dis Primers. 2019;5(1):70. https://doi.org/10.1038/s41572-019-0118-8.
Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639. https://doi.org/10.1161/CIR.0000000000001052.
Singh SN. Costs and clinical consequences of suboptimal atrial fibrillation management. Clinicoecon Outcomes Res. 2012;4:79–90. https://doi.org/10.2147/CEOR.S30090.
Barnes GD, Nallamothu BK, Sales AE, et al. Reimagining anticoagulation clinics in the era of direct oral anticoagulants. Circ Cardiovasc Qual Outcomes. 2016;9(2):182–5. https://doi.org/10.1161/CIRCOUTCOMES.115.002366.
Acknowledgements
Funding
This study (including the Rapid Service and Open Access Fees) was funded by Janssen Scientific Affairs, LLC.
Medical Writing, Editorial, and Other Assistance
Isabelle Ghelerter contributed to the study design and the interpretation of the results for this study and was an employee of Analysis Group, Inc. at the time this study was conducted. Medical writing assistance was provided by Christine Tam, MSc, MWC, an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All named authors have contributed to the conception, study design, and interpretation of the results. All authors revised and approved the final version of the manuscript.
Disclosures
Akshay Kharat and Brahim Bookhart are employees of Janssen Scientific Affairs, LLC, and are stockholders of Johnson & Johnson. Maryia Zhdanava, Dominic Pilon, Gabrielle Caron-Lapointe, and Patrick Lefebvre are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC, which funded the development and conduct of this study and manuscript. Mark Alberts has not received compensation for this project.
Compliance with Ethics Guidelines
Data were de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA) of 1996; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) [26].
Data Availability
The data sets generated and analyzed during the current study are not publicly available because they were used pursuant to a data use agreement. The data are available through requests made directly to Symphony Health, an ICON plc Company.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Alberts, M., Zhdanava, M., Pilon, D. et al. Ischemic Stroke and Systemic Embolism Among One-and-Done Direct Oral Anticoagulant Users with Non-valvular Atrial Fibrillation. Adv Ther 40, 2339–2354 (2023). https://doi.org/10.1007/s12325-023-02483-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02483-4