Abstract
Introduction
The direct oral anticoagulant (DOAC) apixaban has shown to have non-inferior efficacy and better safety than vitamin K antagonists (VKAs) in patients with venous thromboembolism (VTE). We determined whether healthcare resource use (HCRU) and direct all-cause medical and non-medical costs in patients with VTE in France differed between VKAs and apixaban.
Methods
A retrospective cohort study was conducted using French national health data from January 2013–June 2018 for anticoagulant-naïve adults hospitalized with VTE. All-cause costs and HCRU per patient per month (PPPM) were compared between apixaban and VKA cohorts created by 1:1 propensity score matching. Two-part models with bootstrapping were used to calculate marginal effects for costs and HCRU.
Results
The matched VKA and apixaban cohorts each comprised 7503 patients. Compared to VKAs, patients prescribed apixaban had significantly lower (P < 0.0001) mean all-cause costs PPPM for outpatient visits (€438.54 vs. €455.58), overall laboratory tests (€21.26 vs. €83.73), and hospitalizations (€249.48 vs. €343.82), but significantly higher (P < 0.0001) mean all-cause costs PPPM for overall drugs (€97.06 vs. €69.56) and medical procedures (€42.12 vs. €35.50). Mean total all-cause direct medical costs (€687.93 vs. €798.70) and total all-cause direct medical and non-medical costs (€771.60 vs. €883.66) were significantly lower (P < 0.0001) for apixaban. Mean HCRU PPPM showed similar trends. Subgroup analyses showed that, among patients with recurrent VTE, patients prescribed apixaban had significantly lower (P < 0.0001) all-cause costs PPPM for total medical costs (€17.26 vs. €18.12) and total all-cause direct medical and non-medical costs (€18.37 vs. €19.20) than patients prescribed VKAs. Similarly, among patients with bleeding, patients prescribed apixaban had significantly lower (P < 0.0001) all-cause costs PPPM for total medical costs (€15.34 vs. €32.61) and total all-cause direct medical and non-medical costs (€16.23 vs. €34.63) than patients prescribed VKAs.
Conclusion
Compared to VKAs, apixaban may be cost-saving in the treatment of patients hospitalized for acute VTE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
For patients with venous thromboembolism (VTE), the direct oral anticoagulant apixaban is non-inferior to vitamin K antagonists (VKAs) for efficacy and has better safety |
Real-world evidence studies suggest that apixaban is also associated with improved effectiveness |
Healthcare resource use and all-cause costs associated with the use of these anticoagulants in France are not well known |
Our objective was to compare healthcare resource use and all-cause costs between apixaban and VKAs in patients with VTE in France in a real-world setting |
What was learned from the study? |
We found that patients with VTE prescribed apixaban used significantly fewer healthcare resources than patients prescribed VKAs |
Although drug and medical procedure costs were higher for apixaban than for VKAs, apixaban had significantly lower total all-cause medical and non-medical costs than VKAs |
Considering both medical and non-medical costs for VTE patients treated with anticoagulants in France, apixaban saves money compared to VKAs |
Introduction
Venous thromboembolism (VTE) is the formation of a blood clot in a vein and comprises both deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is a leading cause of mortality and morbidity. In Europe, there are nearly 760,000 VTE cases per year [1] In France alone, the number of VTE cases per year is around 120,000 [2]. Moreover, VTE occurred in 1% of hospital stays in 2010–2011 in France [3].
VTE is typically treated with anticoagulants. Traditional anticoagulant treatment for VTE includes parenteral anticoagulants such as heparin agents as well as oral anticoagulants such as the vitamin K antagonist (VKA) warfarin. However, VKAs need regular monitoring to stabilize international normalized ratio (INR) values and frequently have food and drug interactions [5].
Newer anticoagulant treatments include direct oral anticoagulants (DOACs) such as apixaban, which do not require routine monitoring and have fewer food and drug interactions than VKAs [5]. Randomized clinical trials indicate that apixaban and other DOACs have a lower risk of bleeding than VKAs and are non-inferior to VKAs in preventing VTE-related death and VTE recurrence [6, 7]. Several guidelines recommend DOACs (including apixaban) over VKAs as anticoagulation treatment in patients with VTE [8,9,10].
In Europe, aggregate healthcare costs for VTE events are estimated to total up to €13.2 billion per year [11]. An analysis of VKA- and DOAC-associated healthcare costs in 2014–2016 in the USA indicated that the DOAC apixaban had significantly lower all-cause total healthcare costs than warfarin [12]. Also, a Danish cost analysis of patients with VTE indicated that, while drug costs were lower for low molecular weight heparin (LMWH)/VKAs than for DOACs, total costs were lower for DOACs, with apixaban having the lowest total costs among the included DOACs [13]. However, there is generally limited information on whether healthcare costs are different between VKAs and DOACs in France and other European countries.
To properly analyze the risk/benefit balance of different VTE treatment options such as VKAs and DOACs, public health decision makers need HCRU and cost estimates related to VTE treatment. In a recent real-world study using data from the Système National des Données de Santé (SNDS) national health database in France, apixaban showed superior effectiveness and safety compared to VKAs [14]. Using the same study population, the present study aimed to determine whether healthcare resource use (HCRU) and all-cause costs differ between VKAs and apixaban in France. Apixaban is a relevant comparator as it is recommended in treatment guidelines, has demonstrated non-inferiority to VKAs in clinical trials and superiority in real-world studies, and is currently the most frequently prescribed DOAC in France [15]. Additionally, economic evaluation of apixaban was expected to be of interest to decision makers in the public healthcare system in France.
Methods
Overall Study Design and Data Source
This was a retrospective cohort study, designed in collaboration with French healthcare providers (HCPs). The study used data from the SNDS for patients with VTE who were treated with apixaban, VKAs only, or VKAs with LMWH bridging therapy. Patients using VKAs only or VKAs with LMWH bridging were consolidated into a single cohort, hereafter referred to as “VKAs.” The SNDS covers 99% of the French population. SNDS data are linked via unique patient social security numbers (SSNs) and comprise information from primary care, hospital, pharmacy, and death registration databases. The SNDS linked data contain information on patient demographics, medical procedures, causes of death, administered drugs, and hospitalizations. The study period was January 2013–June 2018, reflecting when apixaban was approved and became widely used in France [16].
Study Population
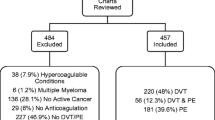
Details of the study population and follow-up are reported elsewhere [14]. Patients were anticoagulant-naïve adults who were covered under French national health insurance, had a VTE event diagnosed during the study period, received ≥ 1 anticoagulant prescription within 30 days after the VTE event discharge date (VKA or apixaban), were hospitalized with VTE, and had no active cancer. This study was approved by the French Institute for Health Data (approval no. 1092905, 23 December 2019). It was conducted with pseudonymized data, as requested by the National Informatics and Liberty Commission (CNIL; approval no. DR-2020-025, 16 January 2020). Informed consent was not required as pseudonymized data were used.
Index VTE Event, Index Therapy, and Index Date
The principal inpatient VTE diagnosis during the study period was designated as the index VTE event. Principal diagnosis was defined as the primary reason the patient was admitted to the hospital. Index therapy was defined as the first anticoagulant to be reimbursed within 30 days after the index VTE event discharge date. The index date for apixaban or VKAs was defined as the date of the first anticoagulant reimbursement that occurred within 30 days after the index VTE event hospital discharge date.
Study Outcomes
Direct healthcare costs in euros were calculated from different types of HCRU. HCRU and related costs were considered all cause and comprised hospital stays (overnight and ambulatory; number of stays, total length of stays in days, and costs), outpatient visits (office-based visits and hospital-based outpatient visits; number and costs), relevant laboratory tests (including INR monitoring) and medical procedures (number and cost), and medications. Hospitalizations included those in short-stay institutions (medicine, surgery, obstetrics), home-based hospitalizations, and hospitalizations related to after care and rehabilitation. Costs reimbursed by health insurance were considered. For hospitalizations, diagnosis-related group (DRG) codes are valued using GHS (groupe homogène de séjours) tariffs (including possible supplements) and therefore reflect reimbursed costs. For medical procedures, reimbursement corresponds to the tariff for the procedure and the social security rate on the date of the procedure [17]. For outpatient consultations, a specific rate is reimbursed by health insurance depending on the complexity of the consultation [18]. For medications, reimbursement is based on official prices on the date of prescription [19]. Total direct medical costs were calculated as the sum of these costs. Total non-medical costs were calculated from sick leave and transportation (which included any type of transportation to an HCP). Total healthcare costs were derived as the sum of direct medical and non-medical costs. Costs were adjusted to 2018 values using the medical component of the consumer price index [20].
In additional subgroup analyses, direct medical and non-medical costs were calculated for patients with recurrent VTE (where the index date was the date of the first recurrent VTE event) and for patients with bleeding (where the index date was the date of the first bleeding event). All patients were included in the analysis, but costs for patients without recurrent VTE or bleeding were considered zero.
Total direct medical and non-medical costs were calculated per patient per month (PPPM) from the index date until 6 months after the index date. For the same period, the most frequent laboratory tests, medical procedures, and drugs prescribed in 2018 (exploratory analysis only) were compared between the apixaban and VKA cohorts by chi-square test. P values < 0.05 were considered significant.
Statistical Analyses
Analyses were conducted using SAS Enterprise Guide version 7.15 (SAS Institute Inc., Cary, NC, USA). Graphs were produced with RStudio Server version 1.2.5042 (RStudio, Boston, MA, USA). Descriptive statistics were calculated for HCRU and for direct medical and non-medical costs; 1:1 propensity score (PS) matching was used to equalize clinical characteristics and baseline demographics between patients administered VKAs and those administered apixaban. Details of this method have been published previously [14].
HCRU and direct medical and non-medical costs were compared between VKAs and apixaban using two-part models. Two-part models are commonly used to model health care data as they are able to accommodate a skewed distribution of data (in the present case, a substantial number of zero observations) [21]. For the main analysis, follow-up started 1 day after the index date and was censored at death, switching of medications, interruption or discontinuation of treatment, end of study, or 6 months after the index date (whichever was earliest). The first part of the model predicted the probability of non-zero HCRU or a non-zero cost (P (y > 0)) using the following formula:
where y was the dependent HCRU or cost and X was the group treated with either anticoagulant. The second part of the model predicted HCRU or costs related to treatment for patients with non-zero HCRU or costs (i.e., patients who used healthcare resources).
A log transformed generalized linear model (GLM) was conducted to estimate predicted HCRU or costs. The final estimated HCRU or cost—the marginal effect (ME)—was calculated using the GENMOD procedure in SAS [22] from the sample means of the data (i.e., it reflects the ME at the mean). The probability of non-zero HCRU or a non-zero cost from the first part of the model was multiplied by the expected HCRU or cost from the second part of the model using the following formula:
where y was the mean HCRU or cost, X was the group treated with either anticoagulant, E was the mean expected value, and E(y|X, y > 0) was the mean predicted HCRU or cost obtained from the second part of the model.
Several GLMs were tested for appropriateness for the second equation. Normal, gamma, Poisson (HCRU only), and negative binomial (HCRU only) distributions were tested. For both HCRU and costs, the gamma distribution had the lowest value for Akaike information criterion (AIC) and was selected for the model; 95% confidence intervals (CIs) were estimated using a bootstrap method with 1000 replications.
Results
Patient Characteristics
Between January 2013 and June 2018, approximately 1.2 million patients with a diagnosis of VTE were identified in the SNDS. A previous publication fully describes the study population [14]. After PS matching, the VKA cohort (N = 7503) and the apixaban cohort (N = 7503) (Supplemental Fig. 1) had similar demographics, comorbidities, and concomitant treatments [14].
HCRU and Costs at 6 Months—Descriptive Statistics
Descriptive statistics were calculated for HCRU PPPM, including outpatient visits, drugs (overall and anticoagulants), laboratory tests (overall and INR), medical procedures, and hospitalizations (number of stays [ambulatory or overnight] and total length of stays in days [overnight only]) (Supplemental Table 1). The most frequent laboratory tests (Supplemental Table 2), medical procedures (Supplemental Table 3), and drugs prescribed in 2018 (Supplemental Table 4) were identified.
Descriptive statistics were also calculated for costs PPPM, including for outpatient visits, drugs (overall and anticoagulants), laboratory tests (overall and INR), medical procedures, hospitalizations, total direct medical costs, and total direct medical and non-medical costs (including sick leave and transportation costs) (Supplemental Table 5). Mean costs PPPM were highest for outpatient visits (€438.48 in the apixaban cohort vs. €455.73 in the VKA cohort) and hospitalizations (€249.34 vs. €343.16).
HCRU and Costs at 6 Months—Comparative Analyses
Marginal Effects
Compared to patients prescribed VKAs, those prescribed apixaban had significantly fewer HCRU events PPPM (mean [95% CI]) for outpatient visits (0.79 [0.79–0.79] vs. 1.28 [1.28–1.28], P < 0.0001), overall laboratory tests (3.77 [3.77–3.78] vs. 10.85 [10.84–10.85], P < 0.0001), number of hospital stays (0.09 [0.09–0.09] vs. 0.12 [0.12–0.12], P < 0.0001), and total length of hospital stays in days (0.67 [0.66–0.67] vs. 0.91 [0.91–0.91], P < 0.0001) (Table 1 and Fig. 1a). Compared to patients prescribed VKAs, those prescribed apixaban had significantly increased HCRU events PPPM (mean [95% CI]) for overall drugs (4.17 [4.17–4.18] vs. 3.16 [3.16–3.16], P < 0.0001) and medical procedures (0.78 [0.78–0.78] vs. 0.65 [0.65–0.65], P < 0.0001).
Compared to patients prescribed VKAs, those prescribed apixaban had significantly lower all-cause costs PPPM (mean [95% CI]) for outpatient visits (€438.54 [438.18–438.90] vs. €455.58 [455.18–455.98], P < 0.0001), overall laboratory tests (€21.26 [21.23–21.28] vs. €83.73 [83.67–83.79], P < 0.0001), hospitalizations (€249.48 [248.93–250.02] vs. €343.82 [342.90–344.73], P < 0.0001), total direct medical costs (€687.93 [687.22–688.65] vs. €798.70 [797.64–799.76], P < 0.0001), and total direct medical and non-medical costs (€771.60 [770.86–772.35] vs. €883.66 [882.57–884.74], P < 0.0001) (Table 2 and Fig. 1b). Compared to patients prescribed VKAs, those prescribed apixaban had significantly higher all-cause costs PPPM (mean [95% CI]) for overall drugs (€97.06 [96.98–97.15] vs. €69.56 [69.36–69.77], P < 0.0001) and medical procedures (€42.12 [42.09–42.15] vs. €35.50 [35.46–35.53], P < 0.0001).
Risk Ratios
Risk ratios for HCRU and all-cause costs showed similar patterns to the marginal effects. Compared to patients treated with VKAs, patients treated with apixaban generally had lower HCRU (Fig. 2a) and lower all-cause costs (Fig. 2b), although costs associated with overall drugs were higher in patients treated with apixaban.
Subgroup Analyses
Descriptive statistics were calculated for total direct medical and non-medical costs PPPM for patients with recurrent VTE (Supplemental Table 6) and patients with bleeding (Supplemental Table 7).
Marginal Effects—Costs for Recurrent VTE at 6 Months
In patients with recurrent VTE, compared to patients prescribed VKAs, those prescribed apixaban had significantly lower all-cause costs PPPM (mean [95%CI]) for total medical costs (€17.26 [17.13–17.40] vs. €18.12 [17.96–18.29], P < 0.0001) and total direct medical and non-medical costs (€18.37 [18.23–18.51] vs. €19.20 [19.03–19.37], P < 0.0001).
Marginal Effects—Costs for Bleeding at 6 Months
In patients with bleeding, compared to patients prescribed VKAs, those prescribed apixaban had significantly lower all-cause costs PPPM (mean [95%CI]) for total medical costs (€15.34 [15.09–15.58] vs. €32.61 [32.13–33.09], P < 0.0001) and total direct medical and non-medical costs (€16.23 [15.99–16.48] vs. €34.63 [34.16–35.10], P < 0.0001).
Discussion
In accordance with previous studies showing superior safety outcomes with apixaban vs. VKAs [7, 14, 23, 24], our results indicate that patients with VTE prescribed apixaban use significantly fewer healthcare resources such as outpatient visits, laboratory tests, and hospital stays (number and total length) than patients prescribed VKAs. The lower HCRU in the apixaban cohort also translated to significantly lower overall costs than in the VKA cohort, with the exception of drug and medical procedure costs, which were higher in the apixaban cohort. As expected, the costs for laboratory tests were much higher in the VKA cohort because of the need for INR monitoring. In the two cohorts, the main drivers of costs were outpatient visits and hospitalizations, which may respectively reflect scheduled follow-up visits after events and hospitalizations related to the events. In patients with VTE recurrence or bleeding, costs were higher in the VKA cohort than in the apixaban cohort.
The high costs of treating recurrent VTE and bleeding events place a burden on the healthcare system. Information on risks and costs of VTE recurrence and bleeding events in VTE patients receiving different anticoagulants is useful to payers in the healthcare system. The risk of VTE after an initial event is 5%–7% and is more than 50 times higher than in patients with no previous VTE events [25]. Additionally, the risk of major bleeding is a concern for patients prescribed anticoagulants [26]. In an observational study using French national claims data in hospitalized adults with VTE based on the same dataset as the current study, patients prescribed apixaban had significantly lower risks of recurrent VTE and bleeding leading to hospitalization than patients prescribed VKAs [14], similar to what was reported in a US claims database study [23].
Similar to the results of the present study, a US Medicare claims study of patients ≥ 65 years with VTE found that patients prescribed warfarin had significantly higher all-cause healthcare and medical costs than patients prescribed apixaban, although recurrent VTE-related medical costs were similar between the cohorts [12]. While both that study and the present study used 1:1 PS matching and had similar numbers of patients, differences in the patient profiles (e.g., age) may have resulted in dissimilarities of recurrent VTE costs. Also similar to the results of our study, a US Medicare claims database analysis of VTE patients with cancer found that patients treated with another DOAC, rivaroxaban, had fewer outpatient visits and hospitalization days than patients treated with LMWH or warfarin [27]. However, in contrast to our study, even though VTE-related costs were lower in the rivaroxaban cohort than in the LMWH cohort, VTE-related costs did not differ between the rivaroxaban and warfarin cohorts [27]. The differences between the findings of that study and the present study could be due to several factors, including the different settings, analysis of different DOACs, and cancer patient status. Our results are also in line with the results of several cost-effectiveness analyses using clinical trial data, which found that apixaban was cost-saving compared to VKAs in patients with acute VTE [28,29,30].
Strengths and Limitations
The strengths of this study include that this was the first comparative study to evaluate HCRU/costs of acute treatment of VTE in France, a country with universal healthcare. While the results of this analysis may not be directly comparable to other countries, they may be representative of other European countries with similar treatment patterns. Moreover, this study should be representative of hospitalized patients with VTE in France, as the French SNDS database includes approximately 99% of the French population. Additionally, the large study population gave high statistical power to detect differences between cohorts. However, the limitations of this study are that the PS matching excluded some patients from the analysis and was thus a potential source of selection bias. The list of variables considered for the PS matching was based on clinical rationale but was dictated by data availability in the SNDS. Clinical measures not available in the SNDS could not be used in PS matching, which may have resulted in unmeasured confounding. Lastly, non-prescription drugs were not included in our analysis as they are not included in the SNDS database. However, we expect that these drugs would have minimal impact on the total costs. Other strengths and limitations of our study design are presented in a previous publication [14].
Conclusions
When allocating healthcare resources for treating acute VTE, decision-makers should consider all key cost drivers of treating the disease and not drug unit costs alone. Mirroring the improved effectiveness and safety results vs. VKAs in real-world studies, apixaban may be cost-saving, despite its higher drug cost, when all direct medical and/or non-medical costs are considered. Based on overall costs, risk of bleeding, and recurrent VTE episodes, the results of our study are in line with published cost-effectiveness analyses and support clinical guidelines recommending physicians and caregivers prescribe apixaban over VKAs.
References
Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. Thromb Haemost. 2007;98(10):756–64.
Bouée S, Emery C, Samson A, Gourmelen J, Bailly C, Cotté FE. Incidence of venous thromboembolism in France: a retrospective analysis of a national insurance claims database. Thromb J. 2016;14:4.
Allaert FA, Benzenine E, Quantin C. Hospital incidence and annual rates of hospitalization for venous thromboembolic disease in France and the USA. Phlebology. 2017;32(7):443–7.
Engbers MJ, van Hylckama VA, Rosendaal FR. Venous thrombosis in the elderly: incidence, risk factors and risk groups. J Thromb Haemost. 2010;8(10):2105–12.
Vranckx P, Valgimigli M, Heidbuchel H. The significance of drug–drug and drug–food interactions of oral anticoagulation. Arrhythm Electrophysiol Rev. 2018;7(1):55–61.
Kakkos SK, Kirkilesis GI, Tsolakis IA. Editor’s choice - efficacy and safety of the new oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban in the treatment and secondary prevention of venous thromboembolism: a systematic review and meta-analysis of phase III trials. Eur J Vasc Endovasc Surg. 2014;48(5):565–75.
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808.
Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–52.
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603.
Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693–738.
Barco S, Woersching AL, Spyropoulos AC, Piovella F, Mahan CE. European Union-28: An annualised cost-of-illness model for venous thromboembolism. Thromb Haemost. 2016;115(4):800–8.
Hlavacek P, Guo JD, Rosenblatt L, Keshishian A, Russ C, Mardekian J, et al. Safety, effectiveness, and health care cost comparisons among elderly patients with venous thromboembolism prescribed warfarin or apixaban in the United States Medicare population. Curr Med Res Opin. 2019;35(12):2043–51.
Nielsen A, Poulsen PB, Dybro L, Kloster B, Lorentzen A, Olsen J, et al. Total costs of treating venous thromboembolism: implication of different cost perspectives in a Danish setting. J Med Econ. 2019;22(12):1321–7.
Bertoletti L, Gusto G, Khachatryan A, Quignot N, Chaves J, Moniot A, et al. Effectiveness and safety of oral anticoagulants in the treatment of acute venous thromboembolism: a nationwide comparative cohort study in France. Thromb Haemost. 2022. https://doi.org/10.1055/a-1731-3922.
Jourdi G, Mansour A, Vayne C, Godon A, Tacquard C, Siguret V, et al. Anticoagulation therapy in France: state-of-the-art in 2020. Ann Blood. 2020;5:3.
Haute Autorité de Santé. ELIQUIS (apixaban), oral anticoagulant 2015. https://www.has-sante.fr/jcms/c_2038860/fr/eliquis-apixaban-anticoagulant-par-voie-orale.
ameli.fr. CCAM en ligne: l'Assurance Maladie; 2022. https://www.ameli.fr/accueil-de-la-ccam/index.php.
ameli.fr. Consultations en métropole : vos remboursements: l'Assurance Maladie. 2022. https://www.ameli.fr/assure/remboursements/rembourse/consultations/metropole.
ameli.fr. Base des Médicaments et Informations Tarifaires: L'Assurance Maladie. 2022. http://www.codage.ext.cnamts.fr/codif/bdm_it/index.php?p_site=AMELI.
Insee. Indice des prix à la consommation–base 2015–ensemble des ménages–France–ensemble: L'Institut national de la statistique et des études économiques, France. 2022. https://www.insee.fr/fr/statistiques/serie/001759970#Telechargement.
Belotti F, Deb P, Manning WG, Norton EC. twopm: two-part models. Stata J. 2015;15(1):3–20.
SAS. GENMOD procedure: SAS Institute. 2022. https://support.sas.com/rnd/app/stat/procedures/genmod.html.
Weycker D, Li X, Wygant GD, Lee T, Hamilton M, Luo X, et al. Effectiveness and safety of apixaban versus warfarin as outpatient treatment of venous thromboembolism in U.S. clinical practice. Thromb Haemost. 2018;118(11):1951–61.
Dawwas GK, Smith SM, Dietrich E, Lo-Ciganic WH, Park H. Comparative effectiveness and safety of apixaban versus warfarin in patients with venous thromboembolism. Am J Health Syst Pharm. 2020;77(3):188–95.
Fahrni J, Husmann M, Gretener SB, Keo HH. Assessing the risk of recurrent venous thromboembolism–a practical approach. Vasc Health Risk Manag. 2015;11:451–9.
Klok FA, Huisman MV. How I assess and manage the risk of bleeding in patients treated for venous thromboembolism. Blood. 2020;135(10):724–34.
Streiff M, Milentijevic D, McCrae KR, Laliberté F, Lejeune D, Lefebvre P, et al. Healthcare resource utilization and costs associated with venous thromboembolism in cancer patients treated with anticoagulants. J Med Econ. 2019;22(11):1134–40.
de Jong LA, Dvortsin E, Janssen KJ, Postma MJ. Cost-effectiveness analysis for apixaban in the acute treatment and prevention of venous thromboembolism in the Netherlands. Clin Ther. 2017;39(2):288-302.e4.
Lanitis T, Leipold R, Hamilton M, Rublee D, Quon P, Browne C, et al. Cost-effectiveness of apixaban versus low molecular weight heparin/vitamin k antagonist for the treatment of venous thromboembolism and the prevention of recurrences. BMC Health Serv Res. 2017;17(1):74.
Lanitis T, Leipold R, Hamilton M, Rublee D, Quon P, Browne C, et al. Cost-effectiveness of apixaban versus other oral anticoagulants for the initial treatment of venous thromboembolism and prevention of recurrence. Clin Ther. 2016;38(3):478-93.e16.
Acknowledgements
Data were provided by the French Caisse Nationale d’Assurance Maladie (CNAM) and its staff involved in the project. Support during the data application process was provided by the Health Data Hub, the Ethics and Scientific Committee for Health Research, Studies and Evaluations (CESREES), and the French Commission Nationale de l’Informatique et des Libertés (CNIL) governing data access and data privacy laws.
Funding
Sponsorship for this study and the Rapid Service and Open Access fees were funded by Pfizer and Bristol Myers Squibb.
Medical Writing Assistance
Medical writing assistance was provided by Holly Richendrfer, PhD, and Stephen Gilliver, PhD, of Evidera and was funded by Pfizer and Bristol Myers Squibb.
Author Contributions
All authors contributed to conceptualization, methodology, formal analysis and investigation, writing—original draft preparation, writing—review and editing, funding acquisition, resources, and supervision.
Disclosures
Ruth Mokgokong, Jose Chaves, and Audrey Moniot are employees and shareholders of Pfizer. Artak Khachatryan, Nadia Quignot and Gaelle Gusto are employees of Certara, which was paid by BMS and Pfizer in connection with the conduct of this study.
Compliance with Ethics Guidelines
This study was approved by the French Institute for Health Data (approval no. 1092905, 23 December 2019). It was conducted with pseudonymized data, as requested by the National Informatics and Liberty Commission (CNIL; approval no. DR-2020–025, 16 January 2020). Informed consent was not required as pseudonymized data were used. This study was performed in accordance with the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mokgokong, R., Khachatryan, A., Quignot, N. et al. Comparative Analysis of All-Cause Health Care Resource Utilization and Costs Among Venous Thrombosis Patients Without Cancer Prescribed Apixaban or VKAs in France. Adv Ther 39, 3766–3776 (2022). https://doi.org/10.1007/s12325-022-02200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02200-7