Abstract
Introduction
The aim was to evaluate the efficacy and tolerability of adjunctive brivaracetam (BRV) in adults with severely drug-resistant focal seizures versus adults with less drug-resistant disease.
Methods
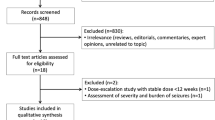
Data were pooled from patients with focal seizures on 1–2 concomitant antiseizure medications (ASMs) randomized to BRV 50, 100, 200 mg/day, or placebo in 3 phase 3 trials (N01252 [NCT00490035], N01253 [NCT00464269], and N01358 [NCT01261325]) with a 12-week treatment period. Outcomes were assessed in patients with ≥ 5 and 0–4 previous ASMs (stopped before trial drug initiation).
Results
In ≥ 5 previous ASMs subgroup (BRV 50, 100, 200 mg/day: n = 26, n = 137, n = 120; placebo: n = 151), percentage reduction over placebo in 28-day adjusted focal seizure frequency was 13.0% for 50 mg/day (p = 0.38), 18.1% for 100 mg/day (p = 0.006), 19.8% for 200 mg/day (p = 0.004), and 17.0% for all BRV-treated patients (p = 0.001). The 50% responder rate was 26.9%, 29.9%, 30.0%, and 29.7% for BRV 50, 100, 200, and 50–200 mg/day, respectively (placebo: 13.2%); odds ratios versus placebo were statistically significant (p < 0.05) for BRV 100, 200, and 50–200 mg/day. In 0–4 previous ASMs subgroup (BRV 50, 100, 200 mg/day: n = 135, n = 195, n = 129; placebo: n = 267), all BRV dosages showed statistically significant (1) percentage reduction over placebo in 28-day adjusted focal seizure frequency (21.4–28.7%); (2) differences from placebo in median percentage reduction in 28-day adjusted focal seizure frequency from baseline (35.5–45.9%; placebo: 21.3%); and (3) odds ratios versus placebo (favoring BRV) for 50% responder rates. In BRV-treated patients, treatment-emergent adverse event (TEAE) incidence (73.8% [217/294] vs. 64.6% [329/509]) and discontinuation due to TEAEs (10.5% vs. 4.5%) were higher in the ≥ 5 versus 0–4 previous ASMs subgroup; serious TEAEs were rare in both subgroups (≥ 5 previous ASMs: 3.1%; 0–4 previous ASMs: 2.9%).
Conclusion
Adjunctive BRV showed efficacy and was generally well tolerated in adults with focal seizures independent of the number of previous ASMs.



Similar content being viewed by others
References
World Health Organization. 2019. Epilepsy. Key facts, https://www.who.int/news-room/fact-sheets/detail/epilepsy. Accessed date 2 Oct 2020
National Institute for Health and Care Excellence (NICE). 2012. Epilepsies: diagnosis and management. Clinical guideline [CG137]. Last updated 11 February 2020, https://www.nice.org.uk/guidance/cg137/chapter/Introduction#pharmacological-treatment. Accessed date 2 Oct 2020
Lynch BA, Lambeng N, Nocka K, et al. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA. 2004;101:9861–6.
Gillard M, Fuks B, Leclercq K, Matagne A. Binding characteristics of brivaracetam, a selective, high affinity SV2A ligand in rat, mouse and human brain: relationship to anti-convulsant properties. Eur J Pharmacol. 2011;664:36–44.
Klitgaard H, Matagne A, Nicolas JM, et al. Brivaracetam: rationale for discovery and preclinical profile of a selective SV2A ligand for epilepsy treatment. Epilepsia. 2016;57:538–48.
Yang X, Bognar J Jr, He T, et al. Brivaracetam augments short-term depression and slows vesicle recycling. Epilepsia. 2015;56:1899–909.
Niespodziany I, Rigo JM, Moonen G, Matagne A, Klitgaard H, Wolff C. Brivaracetam does not modulate ionotropic channels activated by glutamate, gamma-aminobutyric acid, and glycine in hippocampal neurons. Epilepsia. 2017;58:e157–61.
Biton V, Berkovic SF, Abou-Khalil B, Sperling MR, Johnson ME, Lu S. Brivaracetam as adjunctive treatment for uncontrolled partial epilepsy in adults: a phase III randomized, double-blind, placebo-controlled trial. Epilepsia. 2014;55:57–66.
French JA, Costantini C, Brodsky A, von Rosenstiel P, N01193 Study Group. Adjunctive brivaracetam for refractory partial-onset seizures: a randomized, controlled trial. Neurology. 2010;75:519–25.
Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56:1890–8.
Ryvlin P, Werhahn KJ, Blaszczyk B, Johnson ME, Lu S. Adjunctive brivaracetam in adults with uncontrolled focal epilepsy: results from a double-blind, randomized, placebo-controlled trial. Epilepsia. 2014;55:47–56.
Van Paesschen W, Hirsch E, Johnson M, Falter U, von Rosenstiel P. Efficacy and tolerability of adjunctive brivaracetam in adults with uncontrolled partial-onset seizures: a phase IIb, randomized, controlled trial. Epilepsia. 2013;54:89–97.
Ben-Menachem E, Mameniškienė R, Quarato PP, et al. Efficacy and safety of brivaracetam for partial-onset seizures in 3 pooled clinical studies. Neurology. 2016;87:314–23.
Klein P, McLachlan R, Foris K, et al. Effect of lifetime antiepileptic drug treatment history on efficacy and tolerability of adjunctive brivaracetam in adults with focal seizures: post-hoc analysis of a randomized, placebo-controlled trial. Epilepsy Res. 2020;167:106369.
UCB, Inc. 2018. BRIVIACT (brivaracetam) Prescribing Information, https://www.briviact.com/briviact-PI.pdf. Accessed date 2 Oct 2020
Brodie MJ, Barry SJE, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54.
Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75:279–86.
Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology. 2008;70:54–65.
Acknowledgements
The authors thank the patients and their caregivers, in addition to the investigators and their teams who contributed to these trials.
Funding
The trials, the present post hoc analysis, and the journal’s Rapid Service Fee were funded by UCB Pharma (Brussels, Belgium).
Medical Writing, Editorial, and Other Assistance
The authors acknowledge Kyoko Hirano, CMPP (UCB Pharma, Tokyo, Japan) for overseeing publication management, and Svetlana Dimova, MD, PhD (UCB Pharma, Brussels, Belgium), and Connie Hung, PhD (UCB Pharma, Taipei City, Taiwan) for critical review of the manuscript. Writing assistance was provided by Chrysi Petraki (IQVIA, Reading, UK), Tracy-Ann Kernanet-Huggins (IQVIA, Reading, UK), and Laura Huber (IQVIA, Parsippany, NJ, USA) for early drafts, and Emily Chu (Evidence Scientific Solutions, London, UK) for later drafts, and was funded by UCB Pharma.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Sang-Kun Lee, Kyoung Heo, Sung-Eun Kim, and Sang-Ahm Lee were investigators on one or more of the trials. Sami Elmoufti performed the analysis. All authors contributed to the conception and design of the analysis, interpretation of the results, and critical revision of the manuscript for intellectual content. All authors approved the final version of the manuscript for publication.
Disclosures
Sami Elmoufti, Cédric Laloyaux, and Boeun Hur are employees of UCB Pharma. Sang-Kun Lee, Kyoung Heo, Sung-Eun Kim, and Sang-Ahm Lee have no conflicts of interest to declare.
Compliance with Ethics Guidelines
The trials were approved by the appropriate ethical standards committees (USA Central IRB, Copernicus Group IRB; Belgium IRB, Ethics Committee UZA, Universitair Ziekenhuis Antwerpen) and conducted as per applicable regulatory requirements, including International Conference on Harmonisation/Good Clinical Practice requirements, and in accordance with the principles that have their origin in the Declaration of Helsinki. The trials were registered on ClinicalTrials.gov (NCT00490035, NCT00464269, and NCT01261325), and written informed consent to participate was obtained from all patients before enrollment.
Data Availability
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient-level data and redacted trial documents which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Before use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password-protected portal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, SK., Heo, K., Kim, SE. et al. Effect of Number of Previous Antiseizure Medications on Efficacy and Tolerability of Adjunctive Brivaracetam for Uncontrolled Focal Seizures: Post Hoc Analysis. Adv Ther 38, 4082–4099 (2021). https://doi.org/10.1007/s12325-021-01816-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01816-5