Abstract
Introduction
This trial was conducted to assess the long-term safety, efficacy, and benefit of early add-on of linagliptin to insulin in patients with type 2 diabetes mellitus (T2DM).
Methods
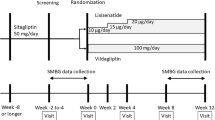
This trial enrolled 246 subjects. The subjects were randomized to the linagliptin group or the control group and were observed for 156 weeks. After week 16, subjects in the control group were also allowed to add linagliptin to evaluate the benefit of early add-on of linagliptin to insulin. The primary end point was a change in HbA1c from baseline to week 16. Secondary end points included fasting plasma glucose, daily insulin dose, and frequency of adverse events.
Results
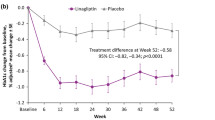
HbA1c and fasting plasma glucose levels significantly decreased from baseline to week 16 in the linagliptin group compared with the control group. The significant improvement in HbA1c continued until week 52. The daily insulin dose significantly decreased in the linagliptin group compared with the control group. The frequency of hypoglycemia and adverse events was comparable in both groups.
Conclusions
Add-on of linagliptin to insulin was tolerated, improved glycemic control, and reduced the daily insulin dose. This study demonstrates the long-term safety, efficacy and benefit of early add-on of linagliptin to insulin in Japanese T2DM patients.


Similar content being viewed by others
References
Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;1:102–9.
Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708.
Ahren B. Dipeptidyl peptidase-4 inhibitors: clinical data and clinical implications. Diabetes Care. 2007;30:1344–50.
Ministry of Health, Labour and Welfare of Japan. The National Health and Nutrition Survey, 2016 (in Japanese). available from. 2017; http://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html. Accessed 31st October, 2017
Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42.
White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–35.
Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26.
Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79.
Kadowaki T, Wang G, Rosenstock J, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA trial. Diabetol Int. 2020;2:2.
Inagaki N, Yang W, Watada H, et al. Linagliptin and cardiorenal outcomes in Asians with type 2 diabetes mellitus and established cardiovascular and/or kidney disease: subgroup analysis of the randomized CARMELINA trial. Diabetol Int. 2020;11:129–41.
Mita T, Katakami N, Shiraiwa T, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the Sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care. 2016;39:455–64.
Mita T, Katakami N, Yoshii H, et al. Alogliptin, a dipeptidyl peptidase 4 inhibitor, prevents the progression of carotid atherosclerosis in patients with type 2 diabetes: the study of preventive effects of alogliptin on diabetic atherosclerosis (SPEAD-A). Diabetes Care. 2016;39:139–48.
Hong D, Si L, Jiang M, et al. Cost effectiveness of sodium-glucose cotransporter-2 (SGLT2) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, and dipeptidyl peptidase-4 (DPP-4) inhibitors: a systematic review. Pharmacoeconomics. 2019;37:777–818.
Geng J, Yu H, Mao Y, Zhang P, Chen Y. Cost effectiveness of dipeptidyl peptidase-4 inhibitors for type 2 diabetes. Pharmacoeconomics. 2015;33:581–97.
Ledesma G, Umpierrez GE, Morley JE, et al. Efficacy and safety of linagliptin to improve glucose control in older people with type 2 diabetes on stable insulin therapy: a randomized trial. Diabetes Obes Metab. 2019;21:2465–73.
Zinman B, Ahren B, Neubacher D, Patel S, Woerle HJ, Johansen OE. Efficacy and cardiovascular safety of linagliptin as an add-on to insulin in type 2 diabetes: a pooled comprehensive post hoc analysis. Can J Diabetes. 2016;40:50–7.
Yki-Jarvinen H, Rosenstock J, Duran-Garcia S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a >/=52-week randomized, double-blind study. Diabetes Care. 2013;36:3875–81.
Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:167–77.
Katsuno T, Ikeda H, Namba M. Medium-term effect of add-on therapy with the DPP-4 inhibitor, sitagliptin, in insulin-treated japanese patients with type 2 diabetes mellitus. Diabetes Ther. 2016;7:309–20.
Katsuno T, Ikeda H, Ida K, Miyagawa J, Namba M. Add-on therapy with the DPP-4 inhibitor sitagliptin improves glycemic control in insulin-treated Japanese patients with type 2 diabetes mellitus. Endocr J. 2013;60:733–42.
Araki E, Unno Y, Tanaka Y, Sakamoto W, Miyamoto Y. Long-term efficacy and safety of linagliptin in a Japanese population with type 2 diabetes aged >/= 60 years treated with basal insulin: a randomised trial. Adv Ther. 2019;36:2697–711.
Graefe-Mody U, Friedrich C, Port A, et al. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin(*). Diabetes Obes Metab. 2011;13:939–46.
von Websky K, Reichetzeder C, Hocher B. Linagliptin as add-on therapy to insulin for patients with type 2 diabetes. Vasc Health Risk Manag. 2013;9:681–94.
McGill JB, Sloan L, Newman J, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: a 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care. 2013;36:237–44.
Ishii H. Development and psychometric validation of the diabetes therapy-related QOL (DTR-QOL) questionnaire. J Med Econ. 2012;15:556–63.
Ishii H, Kim HR, Crawford B. The revalidation of the diabetes treatment-related quality-of-life (DTR-QOL) questionnaire in Japan. Diabetol Int. 2019;10:93–101.
Kawamori R, Inagaki N, Araki E, et al. Linagliptin monotherapy provides superior glycaemic control versus placebo or voglibose with comparable safety in Japanese patients with type 2 diabetes: a randomized, placebo and active comparator-controlled, double-blind study. Diabetes Obes Metab. 2012;14:348–57.
Gallwitz B, Rosenstock J, Rauch T, et al. 2-year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double-blind, non-inferiority trial. Lancet. 2012;380:475–83.
Karagiannis T, Boura P, Tsapas A. Safety of dipeptidyl peptidase 4 inhibitors: a perspective review. Ther Adv Drug Saf. 2014;5:138–46.
Rosenstock J, Kahn SE, Johansen OE, et al. Effect of Linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:2255–1166.
Inzucchi SE, Nauck MA, Hehnke U, Woerle HJ, von Eynatten M, Henry RR. Improved glucose control with reduced hypoglycaemic risk when linagliptin is added to basal insulin in elderly patients with type 2 diabetes. Diabetes Obes Metab. 2015;17:868–77.
Lando HM, Alattar M, Dua AP. Elevated amylase and lipase levels in patients using glucagonlike peptide-1 receptor agonists or dipeptidyl-peptidase-4 inhibitors in the outpatient setting. Endocr Pract. 2012;18:472–7.
Tokuyama H, Kawamura H, Fujimoto M, et al. A low-grade increase of serum pancreatic exocrine enzyme levels by dipeptidyl peptidase-4 inhibitor in patients with type 2 diabetes. Diabetes Res Clin Pract. 2013;100:e66.
Mita T, Hiyoshi T, Yoshii H, et al. The effect of linagliptin versus metformin treatment-related quality of life in patients with type 2 diabetes mellitus. Diabetes Ther. 2019;10:119–34.
Ishii H, Suzaki Y, Miyata Y, Matsui S. Randomized multicenter evaluation of quality of life and treatment satisfaction in type 2 diabetes patients receiving once-weekly trelagliptin versus a daily dipeptidyl peptidase-4 inhibitor. Diabetes Ther. 2019;10:1369–80.
Goto H, Mita T, Fujitani Y, et al. Effects of linagliptin versus voglibose on treatment-related quality of life in patients with type 2 diabetes: sub-analysis of the L-STEP study. Endocr J. 2018;65:657–68.
Acknowledgements
Authors thank Mrs. Kumi Yagi for the collection and management of the clinical data. We thank all the participants in this study. Other members of the TRUST2 study group included H. Seino (Seino Internal Medical Clinic, Fukushima); S. Nakata (Nakata Clinic, Kanagawa); D. Uchida (Hotaruno Central Naika, Chiba), R. Komi, A. Ooka, and A. Tanaka (Shonankamakura General Hospital, Kanagawa); C. Nawa (Shinjuku Mitsui Building Clinic, Tokyo); M. Moriwaki (Moriwaki Clinic, Osaka); F. Haseda (First Towakai Hospital, Osaka); K. Murata (Suisyoukai Murata Hospital, Osaka); Y. Hiromine (Kindai University, Osaka); H. Nishimura (Kumanomae Nishimura Medical Clinic, Tokyo); S. Matsutani, T. Tsunoda, and H. Konya (Ashiya Municipal Hospital, Hyogo); S. Sakane (Hirakata City Hospital, Osaka); H. Ohashi (Oyama East Clinic, Tochigi); A. Nagao, A. Arimitsu, and S. Kurebayashi (Nishinomiya Municipal Central Hospital, Hyogo); H. Fujii (Tama Center Clinic Mirai, Tokyo); H. Imbe, M. Miyawaki, Y. Mishiba, M. Onishi, K. Tanimoto, and F. Nakatsuji (Osaka Medical College, Osaka); J. Kozawa, H. Iwahashi, and K. Fukui (Osaka University, Osaka); T. Yamamoto (Kansai Rosai Hospital, Hyogo); Y. Kojima, A. Aida, and T. Hiyoshi (Japanese Red Cross Medical Center, Tokyo); M. Menjyu (Menjyu Clinic, Osaka); I. Hayashi (Hayashi Clinic, Hyogo).
Funding
This study was financially supported by Boehringer Ingelheim. The journal’s Rapid Service Fee and fee for the technical assistance in the launch and execution of the study and the medical writing of the manuscript by Soiken Inc. were also financially supported by Boehringer Ingelheim.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Tomoyuki Katsuno analyzed the data and wrote, critically reviewed, and approved the final manuscript. Toshihiko Shiraiw, Shingo Iwasaki, Hyohun Park, Nobuaki Watanabe, Shizuka Kaneko, and Jungo Terasaki were involved in the coordination and conduct of the trial and wrote, critically reviewed, and approved the final manuscript. Jun-ichiro Miyagawa, Toshiaki Hanafusa, Akihisa Imagawa, Iichiro Shimomura, Hiroshi Ikegami, Hidenori Koyama, and Mitsuyoshi Namba were involved in the coordination and conduct of the trial and wrote, critically reviewed, and approved the final manuscript.
Medical writing, editorial, and other assistance
The authors thank Soiken Inc. for their technical assistance in the launch and execution of the study and support with the medical writing of the manuscript. This was financially supported by Boehringer Ingelheim.
Disclosures
Toshihiko Shiraiwa has received lecture fees from Takeda Pharmaceutical Co., Ltd., and Sanofi K.K. and grants from Novo Nordisk Pharma Ltd., Kowa Pharmaceutical Co., Ltd., Poxel SA, Daiichi Sankyo Co., Ltd., and Sanofi K.K. SK has received lecture fees from Sumitomo Dainippon Pharma Co., Ltd., Novo Nordisk Pharma Ltd., Eli Lilly and Company, AstraZeneca K.K., Mitsubishi Tanabe Pharma Co., and Boehringer Ingelheim Pharmaceuticals and grants from Eli Lilly and Company, Novo Nordisk Pharma Ltd., Daiichi Sankyo Co., Ltd., and Soiken Inc. Akihisa Imagawa has received lecture fees from Eli Lilly and Company, Ono Pharmaceutical Co., Ltd., and Astellas Pharma Inc. and grants from Astellas Pharma Inc., AstraZeneca K.K., Bristol-Myers Squibb Company, Chugai Pharmaceutical Co., Ltd., Soiken Inc., Taiho Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., and Merck Biopharma Co. Ltd and scholarship contributions from Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., Kyowa Kirin Co., Ltd., and Ono Pharmaceutical Co., Ltd. Iichiro Shimomura has received lecture fees from MSD K.K., Ono Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co., Ltd., Sanofi K.K., Sanwa Kagaku Kenkyusho Co. Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly and Company, and Novo Nordisk Pharma Ltd., grants from Kowa Pharmaceutical Co., Ltd., and ROHTO Pharmaceutical Co., Ltd., and scholarship contributions from Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Teijin Pharma Ltd., Novartis Pharma K.K., and Novo Nordisk Pharma Ltd. Hiroshi Ikegami has received lecture fees from Astellas Pharma Inc., MSD K.K., Terumo Co., Eli Lilly and Company, Novartis Pharma K.K., and Novo Nordisk Pharma Ltd. and scholarship contributions from Takeda Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., LifeScan Japan K.K., Otsuka Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma Ltd., Abbott Japan Co., Ltd., Astellas Pharma Inc., Ono Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Daiichi Sankyo Co., Ltd., Boehringer Ingelheim Pharmaceuticals, Inc., and Bayer Yakuhin Ltd. Hidenori Koyama has received a lecture fee from Sanwa Kagaku Kenkyusho Co. Ltd., grant from AstraZeneca K.K. and Boehringer Ingelheim Pharmaceuticals, Inc., and scholarship contributions from Astellas Pharma Inc., MSD K.K., Ono Pharmaceutical Co., Ltd., Sanofi K.K., Daiichi Sankyo Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Boehringer Ingelheim Pharmaceuticals, Inc., Novo Nordisk Pharma Ltd., and Pfizer Japan Inc. All funding agencies played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Tomoyuki Katsuno, Shingo Iwasaki, Hyohun Park, Nobuaki Watanabe, Shizuka Kaneko, Jungo Terasaki, Toshiaki Hanafusa, Mitsuyoshi Namba, and Jun-ichiro Miyagawa have nothing to disclose.
Compliance With Ethics Guidelines
This study and its protocols were approved by the institutional review board of each participating institution, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Ministry of Health, Labour and Welfare in Japan. This study was registered in the University Hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) (registration number: UMIN000010830). The study was conducted in accordance with the Declaration of Helsinki, Ethical Guidelines for Medical and Health Research Involving Human Subjects as well as other relevant bylaws and regulations. The study protocol was first approved by Hyogo College of Medicine, Independent Ethics Committee, and then other ethics committees for all other institutions. The names of all participated research institutions and the names of ethics committees that have approved the study are listed in supplementary Table 1. Written informed consent was obtained prior to treatment from all enrolled patients who met the eligibility criteria.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the lack of a statement in the study protocol enabling data sharing with a third party after the end of the study and in the informed consent documents as well as lack of approval for data sharing by the institutional review board of each participating institution.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Other members of the TRUST2 study group due listed in the “Acknowledgements” section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Katsuno, T., Shiraiwa, T., Iwasaki, S. et al. Benefit of Early Add-on of Linagliptin to Insulin in Japanese Patients With Type 2 Diabetes Mellitus: Randomized-Controlled Open-Label Trial (TRUST2). Adv Ther 38, 1514–1535 (2021). https://doi.org/10.1007/s12325-021-01631-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-021-01631-y