Abstract
Introduction
The purpose of this study is to investigate the effects of agomelatine on anxious symptoms and functional impairment in a pooled dataset from randomized placebo-controlled trials for generalized anxiety disorder (GAD).
Methods
Data from three randomized, placebo-controlled trials that evaluated the efficacy of agomelatine 25–50 mg were pooled. The short-term (12 weeks) efficacy of agomelatine was assessed in regards to (1) anxious symptoms using the Hamilton Anxiety Scale (HAM-A), and (2) functional impairment using the Sheehan Disability Scale (SDS). Meta-analysis using a random effect model was used to assess differences between groups. Remission and response rates for the HAM-A and SDS were calculated, and analyses were repeated in participants with more severe anxiety symptoms.
Results
In total, 669 patients (340 on agomelatine; 329 on placebo) were included in the analyses. Compared to placebo, the agomelatine group had a significant reduction in HAM-A total score at week 12 (between group difference: 6.30 ± 2.51, p = 0.012). Significant effects were also found for symptom response on the HAM-A (67.1% of patients on agomelatine vs. 32.5% on placebo) and symptom remission (38.8% of patients on agomelatine vs. 17.3% on placebo). Compared to placebo, there was a significant difference in favour of the agomelatine group at week 12 on the SDS total score (5.11 ± 1.81, p = 0.005). Significant effects were also found for functional response on the SDS (79.1% of patients on agomelatine vs. 43.2% of placebo) and functional remission (55.2% of patients on agomelatine vs. 25.4% on placebo). All findings for anxious symptoms and functional impairment were confirmed in the subset of more severely anxious patients. Agomelatine was well tolerated by patients.
Conclusion
This meta-analysis confirms that agomelatine reduces anxiety symptoms and improves the global functioning of GAD patients.
Similar content being viewed by others
This pooled dataset from three short-term randomized placebo-controlled trials for generalized anxiety disorder examines the effects of agomelatine on anxious symptoms and functional impairment of patients. |
On the primary outcome measure of the Hamilton Anxiety Scale, there is a clinically meaningful placebo–agomelatine difference of 6.30 points. |
The data from the Sheehan Disability Scale confirm that agomelatine significantly improves the global functioning of generalized anxiety disorder (GAD) patients. |
The present analysis reinforces evidence for agomelatine’s substantial efficacy in the treatment of anxiety symptoms and functional impairment in GAD, even in severely ill patients. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.13259114.
Supplementary Video 1. (MP4 97804 kb)
Introduction
Generalized anxiety disorder (GAD) is a chronic condition characterized by excessive worry, together with psychic and somatic symptoms of anxiety. GAD is the most common anxiety disorder in primary care practice [1,2,3], and is often associated with both comorbidity (including major depression and other anxiety disorders) and morbidity (including psychosocial impairment and economic costs) [1]. While a number of different medication classes have demonstrated efficacy in the management of GAD and cognitive behavioural therapy remains an important treatment option [4], there is considerable interest in alternative therapies [5,6,7]. Indeed, many patients fail to respond to or cannot tolerate currently recommended pharmacotherapies, such as inhibitors of serotonin and/or noradrenaline reuptake [6]. Additionally, there are significant adverse events associated with the commonly used benzodiazepines, including withdrawal [8].
In contrast to antidepressants, which inhibit monoamine uptake, benzodiazepines that act on the GABAergic system, or buspirone which is also used in GAD management and which behaves as a 5-HT1A partial agonist [6], agomelatine possesses a quite different mechanism of action. It interacts neither with monoamine transporters nor with GABAergic or 5-HT1A receptors, but rather acts as an antagonist at 5-HT2C receptors and as an agonist at melatonin receptor (MT1 and MT2) receptors [9, 10]. This “dual” mechanism of action may underlie its clinical efficacy and “good tolerability” in major depression [9, 11]. Furthermore, with regards to GAD symptoms, antagonism at 5-HT2C receptors may underlie the apparent anxiolytic properties of agomelatine, while melatonin agonism may improve sleep and circadian rhythmicity [9, 12].
Two short-term, double-blind studies and one relapse prevention trial have evaluated the efficacy of agomelatine in the treatment of GAD [13,14,15,16]. In these studies, the efficacy and tolerability of agomelatine in treating GAD were demonstrated using doses of 25–50 mg daily in a placebo-controlled phase II study [14], in a phase III study with escitalopram as active control [16], and in a relapse prevention study [15]. Thereafter, an additional phase III study exploring two doses (10 and 25 mg/day) of agomelatine reinforced early work indicating the efficacy and tolerability of agomelatine 25 mg for the short- and long-term treatment of GAD [17], while a secondary analysis supported the view that this compound is useful for the management of those participants with severe GAD [18]. To the best of our knowledge, and consistent with a recent systematic literature review [19], there are no other randomized placebo-controlled trials investigating the efficacy of agomelatine on GAD.
In the three short-term agomelatine studies, secondary objectives were to assess the potential clinical benefit of agomelatine on symptom response and remission rates, and to provide additional data on the tolerability and safety of agomelatine. As GAD is associated with substantial impairment in functioning and quality of life [20], the Sheehan Disability Scale (SDS) was also included as an outcome [14, 16, 17]. The SDS assesses work/school, social, and family/home functioning [21].
There have been meta-analyses of agomelatine in depression [22,23,24], and of several other agents in GAD [25,26,27,28,29]. So far, one direct meta-analysis has specifically focussed on agomelatine for GAD [30] and a network meta-analysis was performed on 89 randomised trials in outpatients with GAD randomly assigned to 22 different active drugs, including agomelatine [19]. Both meta-analyses included only the two earliest short-term agomelatine studies.
This study reports on a pooled analysis from the three short-term placebo-controlled clinical studies that have used the Hamilton Anxiety Scale (HAM-A) to rate anxiety symptoms and the SDS to measure functional impairment in GAD patients. We also aimed to determine remission and response rates for the HAM-A and SDS, and to repeat analyses in participants with the most severe anxiety symptoms.
Methods
Datasets
Analyses were based on data from three randomized, parallel, double-blind, short-term, placebo-controlled, agomelatine efficacy trials in GAD, conducted by the manufacturer. Efficacy was evaluated using the HAM-A and SDS in adult patients, and with one agomelatine arm in a flexible dosing protocol (agomelatine 25–50 mg) (studies 1 and 2), or with two agomelatine arms with fixed dose (10 or 25 mg) (study 3). In study 1, 63 patients were randomised to receive agomelatine 25–50 mg and 58 to receive placebo. In study 2, 139 patients were randomised to receive agomelatine 25–50 mg, 131 to receive placebo, and 142 to receive escitalopram. In study 3, 131 patients were randomised to receive agomelatine 10 mg (not included in this analysis, as not a therapeutic dose), 139 to receive agomelatine 25 mg (fixed dose), and 142 to receive placebo.
All patients met criteria for a primary diagnosis of GAD according to DSM-IV-TR [31]. The three studies involved a treatment phase of at least 12 weeks and used HAM-A score as the primary outcome measure and the SDS score as a secondary outcome measure. No additional pharmacotherapy or psychotherapy was permitted. Studies were approved by local Ethical Review Boards and were conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
For the purpose of this pooled analysis, only patients treated with placebo or agomelatine at approved therapeutic doses of either 25–50 mg/day (studies 1 and 2) or 25 mg/day (fixed dose, study 3) were considered. In studies 1 and 2, a flexible dosage with up-titration in case of insufficient improvement at week 2 (study 1) or week 4 (study 2), according to a predefined dose adjustment algorithm (25–50 mg/day), was assessed. Both investigators and subjects were blind to the up-titration. Trial registration for each of the studies involved are as follows: Study 1: EudraCT Number, 2004–002577-23; Study 2: Trial registration number, ISRCTN03554974; Study 3: EudraCT Number, 2012–001666-15.
Scales and Assessments
In all three studies, the primary outcome measure was the HAM-A [32], which was rated at the selection and inclusion visits and at weeks 2, 4, 8 and 12. Anxiolytic efficacy over 12 weeks was assessed using the last post-baseline value on the total score of the HAM-A scale. Symptom response (at least 50% decrease from baseline on the HAM-A total score) and symptom remission (HAM-A total score ≤ 7), were secondary outcome measures.
Functional impairment was assessed using the well-validated self-rated SDS [21], which measures the impact of symptoms on work/school, social life, and family life/home responsibilities. The SDS total score is the sum of the three domain scores and ranges from 0 to 30. Following treatment, a total score of 12 or less is considered a “functional response”, while a total score of 6 or less is a good indicator of ‘’functional remission’’ [33].
Safety measures included adverse events reporting at each visit, vital signs (heart rate, blood pressure), standard laboratory tests (biochemistry, haematology), including liver function tests (ALT, AST, γ-GT, ALP and total bilirubin). All safety measures were also performed in the case of premature withdrawal.
Subjects
Eligible patients were required to have a HAM-A total score of ≥ 22, with a score of ≥ 2 on both HAM-A items 1 (anxious mood) and 2 (tension), a Hospital and Depression Anxiety [34] score ≥ depression score, and a Montgomery–Åsberg Depression Rating Scale [35] score of ≤ 16 at selection and inclusion. For studies 2 and 3, HAM-A items 1 + 2 ≥ 5 was required at selection and inclusion. Patients with a decrease of greater than 20% on the HAM-A scale between selection and inclusion were excluded. All patients were required to be physically healthy or to have stabilised somatic illness. Exclusion criteria have been described previously [14, 16, 17].
Statistical Analyses
Baseline characteristics were recorded, and efficacy analyses were performed in the Full Analysis Set (FAS, included all randomized patients who took at least one dose of medication, with ratings at baseline and at least one post-baseline visit for the primary efficacy criterion). Missing data were imputed with the Last Observation Carried Forward approach for all post-baseline criteria.
Agomelatine–placebo difference in HAM-A total score at endpoint was examined in the FAS using analysis of variance. Meta-analysis was employed to compute the overall treatment effect using a random effect model. Meta-analyses using a random effect model were also performed for symptom response and remission rates at endpoint. These analyses were repeated in the subset of patients with HAM-A total score ≥ 25 at baseline.
Agomelatine–placebo differences were also assessed at endpoint in the FAS for SDS work/school, social, and family/home functioning scores, and total score, using an analysis of variance. Meta-analyses using a random effect model were employed for SDS scores, and functional response and remission rates, as defined by a total SDS score of 12 or less and by a a total SDS score of 6 or less respectively to estimate the overall treatment effect. These analyses were again repeated in the subsets of patients with HAM-A total score ≥ 25 at baseline.
For all the meta-analyses, the Cochran homogeneity test, I2 degree of inconsistency, and forest plots were used to assess the homogeneity between treatment effects.
For each safety measurement, descriptive statistics were provided by treatment group in the safety set, defined as all included patients having taken at least one dose of study medication.
Statistical analyses were performed on SAS® software, v.9.2 (Cary, NC, USA). The type I error was set at 5% (two-sided tests).
Results
Demographics and Baseline Characteristics
A total of 669 patients (340 treated with agomelatine 25–50 mg and 329 treated with placebo) were included in the analyses. The mean age at selection was 43.7 ± 13.5 and 42.9 ± 12.7 years (mean ± SD) in the agomelatine and placebo groups, respectively; there were no clinically relevant differences between the groups on demographic or clinical characteristics (Table 1). Among the 340 patients treated with agomelatine, 44 (12.9%) had a dose increase at week 2 or 4.
A total of 107 patients did not complete the trial (completer rate 84%), including 40 patients in the agomelatine group and 67 patients in the placebo group. Reasons for withdrawal were mainly lack of efficacy (18 patients in the agomelatine group and 45 patients in the placebo group) and non-medical reasons (12 patients in the agomelatine group and 14 patients in the placebo group).
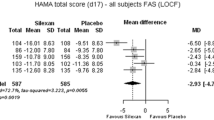
Anxiety Symptoms (Fig. 1; Tables 2 and 3)
Forest plot of the HAM-A total score difference in the FAS. The heterogeneity between studies was mainly related to effect size, indicating a quantitative interaction between treatment effect and study. An estimate of overall treatment effect can be interpreted since the random-effects model considered such heterogeneity
In the whole study population, the treatment with agomelatine was associated with a statistically significant greater reduction in HAM-A score than was placebo: 6.30 ± 2.51 points at the last post-baseline value (p = 0.012). The Cochran homogeneity test was statistically significant (p < 0.001) with an I2 degree of inconsistency of 91.67% (scored 0–100, with a higher score indicating smaller homogeneity).
Symptomatic response (defined by at least a 50% decrease from baseline on HAM-A total score) was achieved by 228 out of 340 patients (67.1%) in the agomelatine group, compared to 107 out of 329 patients (32.5%) in the placebo group, with a significant difference of 32.6 ± 8.4% (p < 0.001). The number needed to treat (NNT, calculated using the estimate of the difference from the meta-analysis) was 3.1 for symptomatic response. The number of patients with a HAM-A total score ≤ 7 at last post-baseline value, indicating symptomatic remission, was 132 out of 340 (38.8%) in the agomelatine group versus 57 out of 329 (17.3%) in the placebo group with a significant difference of 21.67 ± 3.40% (p < 0.001). The NNT was 4.6 for symptomatic remission.
HAM-A total scores and HAM-A responses at each visit of are provided in Table 4. Notably, at W4, 30.21% of patients in agomelatine were responders compared to 14.95% of patients in placebo.
More Severely Anxious Patients (Table 4)
For patients with HAM-A total score at baseline ≥ 25 (n = 587; 87.7% of the whole population), the superiority of agomelatine versus placebo was established with an unadjusted difference on last post-baseline value in HAM-A total score of 6.49 ± 2.71 points (p = 0.017). Response rates were 67.6% for agomelatine and 31.6% for placebo with a significant difference of 33.36 ± 8.40% (95% CI 14.62; 52.09, p < 0.001). The NNT was 3.0 for symptomatic response in the more severely anxious subset. Remission rates were 38.2% on agomelatine and 17.5% on placebo with a significant difference of 20.47 ± 4.49% (95% CI 11.68; 29.26, p < 0.001). The NNT was 4.9 for symptomatic remission in the more severely anxious subset.
Functional Impairment
The Cochran homogeneity test was statistically significant (p < 0.001) and the I2 degree of inconsistency was 88.33%. However, the forest plot showed that the treatment effect on the SDS total score at endpoint was in favor of agomelatine in each of the studies (Fig. 2). The last post-baseline value of the total SDS score was significantly lower in the agomelatine group than in the placebo group (Table 5); placebo–agomelatine differences were 5.11 ± 1.81 points (p = 0.005). There was a statistically significant difference in favour of agomelatine versus placebo on each of the three subscales: for work functioning, placebo–agomelatine difference was 1.73 ± 0.59 points, p = 0.004; for social functioning placebo–agomelatine difference was 1.74 ± 0.64 points, p = 0.006 and for family functioning placebo–agomelatine difference was 1.70 ± 0.63 points, p = 0.007.
Forest plot of SDS total score difference in the FAS. The heterogeneity between studies was mainly related to effect size, indicating a quantitative interaction between treatment effect and study. An estimate of overall treatment effect can be interpreted since the random-effects model considered such heterogeneity
Functional response (defined as a SDS total score ≤ 12) was obtained in 219 out of 277 patients (79.1%) in the agomelatine group, compared to 121 out of 280 (43.2%) in the placebo group, with a significant placebo–agomelatine difference (33.4 ± 9.16%, p < 0.001) (Table 6). The NNT was 3.0 for functional response. Functional remission (defined as a SDS total score ≤ 6) was obtained by 153 out of 277 patients (55.2%) in the agomelatine group versus 71 out of 280 (25.4%) in the placebo, with a significant placebo–agomelatine difference of 29.5 ± 5.1% (p < 0. 001) (Table 6). The NNT was 3.4 for functional remission.
Descriptive statistics at each visit of SDS total score (Table 2) are provided for the W0–W12 period.
More Severely Anxious Patients
For patients with HAM-A total score at baseline ≥ 25, the last post-baseline value of the total SDS score was significantly lower in the agomelatine group than in the placebo group with a placebo–agomelatine difference of 5.24 ± 1.97 points (95% CI 1.37; 9.11, p = 0.008). Significant differences in favour of agomelatine versus placebo were found on all subscales: for work functioning, the placebo–agomelatine difference was 1.78 ± 0.64 points (95% CI 0.53; 3.02, p = 0.005); for social functioning, the placebo–agomelatine difference was 1.80 ± 0.68 points (95% CI 0.46; 3.13, p = 0.008), and for family/home functioning, the placebo–agomelatine difference was 1.77 ± 0.67 points (95% CI 0.46; 3.07, p = 0.008).
Functional response was obtained in 187 out of 241 patients (77.6%) in the agomelatine group versus 101 out of 245 patients (41.2%) in the placebo group; a significant placebo–agomelatine difference (33.17 ± 10.98%, 95% CI 11.65; 54.69, p = 0.003). The NNT was 3.0 for functional response in the more severely anxious subset. Functional remission was obtained in 131 out of 241 patients (54.4%) in the agomelatine group versus 58 out of 245 patients (23.7%) in the placebo group, with a significant placebo–agomelatine difference (30.56 ± 4.60%, 95% CI 21.55; 39.57, (p < 0.001). The NNT was 3.3 for functional remission in the more severely anxious subset.
Safety
In the safety set (n = 670), similar percentages of patients reported at least one emergent adverse event (EAE) during the 12-week treatment period in agomelatine 25–50 mg (44.3%) and placebo (41.3%) groups (Table 7). The three most frequent EAEs on agomelatine were headache, nasopharyngitis and nausea. A total of eight patients in the agomelatine group (2.4%) reported at least one severe emergent adverse event. Adverse events lead to treatment discontinuation for seven patients in the agomelatine 25 mg group (2.1%) and seven patients in the placebo group (2.1%). EAEs which led to a treatment withdrawal were AST, ALT (both predefined withdrawal criteria) and GGT increase, gastrointestinal disorders, and headache in the agomelatine 25 mg group, and neck pain and psychiatric and sleep disorders in the placebo group.
Serious EAEs (SEAEs) were reported by four patients (1.2%) in the agomelatine 25–50 mg (all patients were on agomelatine 25 mg), and by four patients (1.2%) in the placebo group. The most frequent SEAEs in the agomelatine group were AST and ALT increase (two patients on agomelatine 25 mg). One SEAE on agomelatine (AST and ALT increase) was considered treatment-related but did not lead to study drug withdrawal and resolved.
There were no clinically relevant differences between groups, nor changes from baseline to the last value on treatment, in any set, with regard to vital signs and biochemical and haematological parameters.
Six patients in the agomelatine group (1.8%) had emergent potentially clinically significant abnormal (PCSA) transaminases at week 12. Two patients on agomelatine 25 mg presented with ALT or AST in the range of 3–5 × the upper limit of normal (ULN), three patients (two on agomelatine 25 mg and one on agomelatine 50 mg) presented with ALT or AST in the range of 5–10 × ULN, and one patient on agomelatine 25 mg presented with ALT or AST > 10 × ULN. All values normalized after study drug discontinuation.
Discussion
These results quantify the efficacy of agomelatine 25–50 mg in the short-term treatment of GAD symptoms, and of GAD symptoms in more severely anxious individuals. The meta-analysis found that, on the primary outcome measure of the HAM-A, there is a clinically meaningful placebo–agomelatine difference of 6.30 points. This is supported by the high rates of response (67.1%) and remission (38.8%). These findings are in line with those from the pooling of other randomized clinical trials of SSRI/SNRIs for GAD [36,37,38]. They are also consistent with the recent results of a systematic review and network meta-analysis showing that, among a large panel of pharmacological agents, agomelatine has good efficacy with established tolerability, as long as it is not associated with hepatic effects [19]. Furthermore, compared to a previous meta-analysis of agomelatine trials in GAD [30], we found a higher placebo–agomelatine difference, due to the incorporation of an additional recent study [17].
As most patients (87.7%) in the population studied were rated as severely anxious at baseline (HAM-A total score > 25), results of agomelatine efficacy were roughly the same in this subset of patients, with a 6.49-point difference versus placebo on the HAM-A and substantial rates of response (67.6%) and remission (38.2%). When severe, GAD symptoms in patients are often associated with significant social and occupational impairment [2, 39]. Severe GAD is associated with increased risk for suicidality [40], and lower response rates to intervention [8]. Nevertheless, there have been few pharmacotherapy trials for severe GAD [18, 41, 42]. The present findings, obtained in an appropriately powered sample of GAD patients with severe GAD, are consistent with a recent trial of agomelatine in this population [18], and indicate that this medication is useful for reducing anxiety symptoms and functional impairment in these clinically vulnerable patients.
Effect sizes, as measured by NNT, were also noteworthy. The NNTS for agomelatine HAM-A response and remission were 3.1 and 4.6 in the full GAD analysis set and 3.0 and 4.9 in the more severe GAD subset. To put these effect sizes in context, they are at least as good as those found in antidepressant medication analyses in acute major depressive disorder trials. While this meta-analysis focused on outcomes at 12 weeks, data obtained at the assessed visits suggest an improvement of symptoms from week 2 (See Table 2). Notably, statistically significant differences to placebo were observed from week 2 in one of the phase III studies [17] and from week 4 in another [16] (unpublished data).
Patients hold that remission requires not only symptomatic remission but also normalization of functioning, [43] and indeed recovery of GAD patients can be defined in terms of both symptom severity and global functioning [44]. Disability assessment and functional outcome measures have increasingly been recognized as important in clinical practice and in clinical trials. Four scales are frequently used to reliably measure patients’ functioning and disability: the self-rated SDS [21]; the Social Adjustment Scale–Self Report [45]; the Social Adaptation Self-Evaluation Scale [46] and the Medical Outcome Study SF-36 [47]. These scales vary from those requiring a structured interview with a trained assessor to self-assessment scales, and a balance must be found between obtaining enough detail to produce a clear account of global functioning and ease of use in large multicentre studies. The fully validated self-rated SDS was specifically chosen in all trials of agomelatine for GAD because: (1) it is the most widely used scale for assessing functional impairment in psychiatry; (2) it has demonstrated sensitivity in differentiating medications from placebo; and (3) it has been recommended as the most relevant and easy to use self-reported assessment of global functioning in trials of antidepressants [48].
The present pooled results of SDS scores show that agomelatine significantly improves patient functioning after 12 weeks of treatment. The mean last post-baseline value in agomelatine in the SDS total score was 7.6 ± 6.7, with a significant difference of 5.11 points over placebo at endpoint. The uniform improvement on all three domains of function is clinically meaningful, particularly given the short-term endpoint. Few studies of antidepressants in GAD have used the SDS to determine ‘functional response’ as defined by a SDS total score ≤ 12, and ‘functional remission’—based on a SDS total score ≤ 6-—of patients on treatment [33]. In our analyses, more than three-quarters of patients (79.1%) showed such a ‘functional response’ and more than half (55.2%) of patients showed ‘functional remission’ after short-term treatment. The benefit of agomelatine on global functioning was also apparent in the subset of more severely anxious patients, with similar effects on SDS scores and rates of functional response and remission. Clinically meaningful improvement in a range of domains is consistent with previous work on agomelatine [49,50,51,52].
As individuals with GAD often experience significantly reduced quality of life in many areas (general physical and mental health, role functioning due to physical and emotional difficulties, and social functioning) [1] so rapid and effective treatment of anxiety symptoms might translate into substantially improved global functioning [20]. The experience of decreased symptoms and increased functioning, together with the favourable adverse effect profile of agomelatine, may lead to greater adherence to treatment. Although agomelatine is not, at present, indicated for the treatment of generalized anxiety disorder, these data reinforce evidence for the efficacy and tolerability of agomelatine in GAD, including in those with more severe GAD symptoms.
Several limitations deserve emphasis. First, several exclusion criteria were used in each of the three trials, which limits the generalizability of these findings to patients presenting in routine clinical practice, where GAD is characterized by comorbidity with depression or other anxiety disorders [53]. However, most enrolled patients had severe GAD symptoms and high levels of associated disability, and such patients may be more representative of the population seen in clinical practice. Second, the self-rated SDS was administered at 12 weeks, and there is a need to supplement this work with clinician-rated instruments given over the long term in order to fully understand functional impairment associated with GAD in naturalistic settings. However, it is notable that improved global functioning of GAD patients was observed over a long-term period in the agomelatine relapse prevention study [15], while a 6-month placebo-controlled agomelatine study also demonstrated a robust restoration of global functioning in MDD at this time point [51]. Third, the Cochrane homogeneity test was found to be significant, and, in addition to a high I2 degree of inconsistency, may indicate heterogeneity of the data. However, forest plots suggested that heterogeneity was mainly related to effect size, indicating a quantitative interaction between treatment effect and study. An estimate of overall treatment effect can be made since the random-effects model addresses such heterogeneity. A final limitation is the use of editorial assistance in preparation of the manuscript, sponsored by the pharmaceutical company whose product the study reviews. However, the first author contributed significantly to the actual writing of the manuscript, all other authors participated in reviewing and revising the manuscript, and authors have been transparent in listing potential biases.
Conclusion
The present analysis reinforces evidence for the substantial and rapid efficacy of agomelatine in treatment of anxiety symptoms and functional impairment in GAD. There is a need for further work to consolidate these observations and, employing rigorous and pragmatic research designs, to explore the efficacy and utility of agomelatine in anxiety disorders other than GAD. The findings here indicate that the overall risk for serious adverse events for agomelatine may be equivalent to placebo, although there have been concerns about potential hepatic impact given increased risk of transaminase elevations [54, 55]. Given its established efficacy, safety and tolerability, agomelatine may be particularly useful for reducing the distressing symptoms and functional impairment seen in severely ill patients with GAD. Further clinical research on symptom reduction and on quality of life improvement in GAD is important given that functional remission is an important criterion of recovery.
References
Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25(1):72–90.
Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–79.
Parmentier H, Garcia-Campayo J, Prieto R. Comprehensive review of generalized anxiety disorder in primary care in Europe. Curr Med Res Opin. 2013;29(4):355–67.
Bandelow B, Lichte T, Rudolf S, Wiltink J, Beutel ME. The diagnosis of and treatment recommendations for anxiety disorders. Dtsch Arztebl Int. 2014;111(27–28):473–80.
Bui E, King F, Melaragno A. Pharmacotherapy of anxiety disorders in the 21st century: a call for novel approaches. Gen Psychiatr. 2019;32(6):e100136.
Craske MG, Stein MB, Eley TC, et al. Anxiety disorders. Nat Rev Dis Primers. 2017;3:17024.
Schanzer B, Rivas-Grajales AM, Khan A, Mathew SJ. Novel investigational therapeutics for generalized anxiety disorder (GAD). Expert Opin Investig Drugs. 2019;28(11):1003–12.
Stein MB, Sareen J. Clinical practice. Generalized anxiety disorder. N Engl J Med. 2015;373(21):2059–68.
de Bodinat C, Guardiola-Lemaitre B, Mocaer E, Renard P, Munoz C, Millan MJ. Agomelatine, the first melatonergic antidepressant: discovery, characterization and development. Nat Rev Drug Discov. 2010;9(8):628–42.
Millan MJ, Gobert A, Lejeune F, et al. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine(2C) receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther. 2003;306(3):954–64.
Guardiola-Lemaitre B, Bodinat C, Delagrange P, Millan MJ, Munoz C, Mocaer E. Agomelatine: Mechanism of action and pharmacological profile in relation to antidepressant properties. Br J Pharmacol. 2014;171:3604–19.
Millan MJ, Brocco M, Gobert A, Dekeyne A. Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade. Psychopharmacology. 2005;177(4):448–58.
Levitan MN, Papelbaum M, Nardi AE. Profile of agomelatine and its potential in the treatment of generalized anxiety disorder. Neuropsychiatr Dis Treat. 2015;11:1149–55.
Stein DJ, Ahokas AA, de Bodinat C. Efficacy of agomelatine in generalized anxiety disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(5):561–6.
Stein DJ, Ahokas AA, Albarran Severo C, Olivier V, Allgulander C. Agomelatine prevents relapse in generalised anxiety disorder: a 6-month placebo-controlled discontinuation study. J Clin Psychiatry. 2012;73(7):1002–8.
Stein DJ, Ahokas A, Marquez MS, et al. Agomelatine in generalized anxiety disorder: an active comparator and placebo-controlled study. J Clin Psychiatry. 2014;75(4):362–8.
Stein DJ, Ahokas A, Jarema M, et al. Efficacy and safety of agomelatine (10 or 25 mg/day) in non-depressed out-patients with generalized anxiety disorder: a 12-week, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2017;27(5):526–37.
Stein DJ, Khoo JP, Ahokas A, et al. 12-week double-blind randomized multicenter study of efficacy and safety of agomelatine (25–50mg/day) versus escitalopram (10–20mg/day) in out-patients with severe generalized anxiety disorder. Eur Neuropsychopharmacol. 2018;28(8):970–9.
Slee A, Nazareth I, Bondaronek P, Liu Y, Cheng Z, Freemantle N. Pharmacological treatments for generalised anxiety disorder: a systematic review and network meta-analysis. Lancet. 2019;393(10173):768–77.
Wilson H, Mannix S, Oko-osi H, Revicki DA. The impact of medication on health-related quality of life in patients with generalized anxiety disorder. CNS Drugs. 2015;29(1):29–40.
Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(Suppl 3):89–95.
Guaiana G, Gupta S, Chiodo D, Davies SJ, Haederle K, Koesters M. Agomelatine versus other antidepressive agents for major depression. Cochrane Database Syst Rev. 2013;12:CD008851.
Kennedy SH, Grouin JM, Cadour S, Robert V, Picarel-Blanchot F. Relative short-term efficacy and acceptability of agomelatine versus vortioxetine in adult patients suffering from major depressive disorder. Hum Psychopharmacol. 2018;238:122–8.
Taylor D, Sparshatt A, Varma S, Olofinjana O. Antidepressant efficacy of agomelatine: meta-analysis of published and unpublished studies. BMJ. 2014;348:g1888.
Generoso MB, Trevizol AP, Kasper S, Cho HJ, Cordeiro Q, Shiozawa P. Pregabalin for generalized anxiety disorder: an updated systematic review and meta-analysis. Int Clin Psychopharmacol. 2017;32(1):49–55.
Gomez AF, Barthel AL, Hofmann SG. Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin Pharmacother. 2018;19(8):883–94.
Li X, Zhu L, Su Y, Fang S. Short-term efficacy and tolerability of venlafaxine extended release in adults with generalized anxiety disorder without depression: a meta-analysis. PLoS ONE. 2017;12(10):e0185865.
Li X, Zhu L, Zhou C, et al. Efficacy and tolerability of short-term duloxetine treatment in adults with generalized anxiety disorder: a meta-analysis. PLoS ONE. 2018;13(3):e0194501.
Zareifopoulos N, Dylja I. Efficacy and tolerability of vilazodone for the acute treatment of generalized anxiety disorder: A meta-analysis. Asian J Psychiatr. 2017;26:115–22.
Demyttenaere K. Agomelatine in treating generalized anxiety disorder. Expert Opin Investig Drugs. 2014;23(6):857–64.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edn (DSM-IV-TR). 4th ed. Washington DC: American Psychiatric Association; 2000.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–5.
Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23(2):70–83.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Goodman WK, Bose A, Wang Q. Treatment of generalized anxiety disorder with escitalopram: pooled results from double-blind, placebo-controlled trials. J Affect Disord. 2005;87(2–3):161–7.
Meoni P, Hackett D, Lader M. Pooled analysis of venlafaxine XR efficacy on somatic and psychic symptoms of anxiety in patients with generalized anxiety disorder. Depress Anxiety. 2004;19(2):127–32.
Pae CU, Wang SM, Han C, et al. Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res. 2015;64:88–98.
Kessler RC, Brandenburg N, Lane M, et al. Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychol Med. 2005;35(7):1073–82.
Norton PJ, Temple SR, Pettit JW. Suicidal ideation and anxiety disorders: elevated risk or artifact of comorbid depression? J Behav Ther Exp Psychiatry. 2008;39(4):515–25.
Matza LS, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19(4):223–32.
Liebowitz MR, DeMartinis NA, Weihs K, et al. Efficacy of sertraline in severe generalized social anxiety disorder: results of a double-blind, placebo-controlled study. J Clin Psychiatry. 2003;64(7):785–92.
Zimmerman M, McGlinchey JB, Posternak MA, Friedman M, Attiullah N, Boerescu D. How should remission from depression be defined? The depressed patient’s perspective. Am J Psychiatry. 2006;163(1):148–50.
Stein DJ, Bandelow B, Dolberg OT, Andersen HF, Baldwin DS. Anxiety symptom severity and functional recovery or relapse. Ann Clin Psychiatry. 2009;21(2):81–8.
Gameroff MJ, Wickramaratne P, Weissman MM. Testing the Short and Screener versions of the Social Adjustment Scale-Self-report (SAS-SR). Int J Methods Psychiatr Res. 2012;21(1):52–65.
Bosc M, Dubini A, Polin V. Development and validation of a social functioning scale, the Social Adaptation Self-evaluation Scale. Eur Neuropsychopharmacol. 1997;7(Suppl 1):S57–70.
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83.
Bech P. Social functioning: should it become an endpoint in trials of antidepressants? CNS Drugs. 2005;19(4):313–24.
Heun R, Ahokas A, Boyer P, Giménez-Montesinos N, Pontes-Soares F, Olivier V. The efficacy of agomelatine in elderly patients with major recurrent depressive disorder: a placebo controlled study. J Clin Psychiatry. 2013;74(6):587–94.
Kennedy SH, Avedisova A, Gimenez-Montesinos N, Belaidi C, de Bodinat C. A placebo-controlled study of three agomelatine dose regimens (10mg, 25mg, 25–50mg) in patients with major depressive disorder. Eur Neuropsychopharmacol. 2014;24(4):553–63.
Kennedy SH, Avedisova A, Belaidi C, Picarel-Blanchot F, de Bodinat C. Sustained efficacy of agomelatine 10 mg, 25 mg, and 25–50 mg on depressive symptoms and functional outcomes in patients with major depressive disorder. A placebo-controlled study over 6 months. Eur Neuropsychopharmacol. 2016;26(2):378–89.
Udristoiu T, Dehelean P, Nuss P, Raba V, Picarel-Blanchot F, de Bodinat C. Early effect on general interest, and short-term antidepressant efficacy and safety of agomelatine (25–50mg/day) and escitalopram (10–20mg/day) in outpatients with Major Depressive Disorder. A 12-week randomised double-blind comparative study. J Affect Disord. 2016;199:6–12.
Hoertel N, Le SY, Blanco C, Lavaud P, Dubertret C. Generalizability of clinical trial results for generalized anxiety disorder to community samples. Depress Anxiety. 2012;29(7):614–20.
Pladevall-Vila M, Pottegard A, Schink T, et al. Risk of acute liver injury in agomelatine and other antidepressant users in four European countries: a cohort and nested case-control study using automated health data sources. CNS Drugs. 2019;33(4):383–95.
Voican CS, Corruble E, Naveau S, Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. 2014;171(4):404–15.
Acknowledgements
The authors thank all participating investigators in the three studies.
Funding
This study and the journal's Rapid Service and Open Access Fees were funded by Servier.
Medical Writing and Editorial Assistance
A medical writing assistant (Pierre-Alain Boyer, Calliopae), funded by Servier, was used in preparation of the manuscript. Mark J Millan helped with parts of the manuscript.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Pr. Dan Stein has received research grants and/or consultancy honoraria from Lundbeck, Servier, and Johnson & Johnson. Pr Jon-Paul Khoo has been funded for research, participated in Consultancy Panels/Advisory Boards, received Educational grants or received speaking honoraria from Alkermes; AstraZeneca; Biomonics; Bristol-Myers Squibb; Eli Lilly; GlaxoSmithKline; Janssen; Lundbeck; Pfizer; Sanofi-Aventis; Servier; and/or Wyeth. Pr Jon-Paul Khoo has no financial interest in pharmaceutical companies, and receives no royalties, stock options, or equity in this regard. Pr Michael Van Ameringen has received research grants and/or consultancy honoraria from Allergan, Almatica, Brainsway, Janssen-Ortho Inc., Lundbeck, Myriad Neuroscience, Otsuka, Pfizer, Purdue Pharma (Canada), the Canadian Foundation for Innovation and Hamilton Academic Health Sciences Organization (HAHSO). Valérie Olivier and Françoise Picarel-Blanchot are employees at Servier.
Compliance With Ethics Guidelines
Studies were approved by local Ethical Review Boards and were conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Stein, D.J., Khoo, JP., Picarel-Blanchot, F. et al. Efficacy of Agomelatine 25–50 mg for the Treatment of Anxious Symptoms and Functional Impairment in Generalized Anxiety Disorder: A Meta-Analysis of Three Placebo-Controlled Studies. Adv Ther 38, 1567–1583 (2021). https://doi.org/10.1007/s12325-020-01583-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01583-9