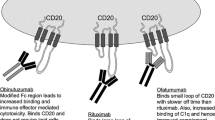
Abstract
Rituximab (Rituxan®; iogen Idec, San Diego, CA, USA) is a human-mouse chimeric monoclonal antibody specific for CD20, a surface glycoprotein expressed on B lymphocytes. Administration of rituximab as a single agent to patients with chronic lymphocytic leukemia (CLL) has limited clinical activity, but generally does not eradicate leukemia from the marrow. However, when administered in combination with chemotherapy, rituximab can improve the survival of patients relative to those treated with chemotherapy alone. As a result of this, the US Food and Drug Administration approved the use of rituximab in previously untreated and previously treated CD20-positive CLL in combination with fludarabine monophosphate and cyclophosphamide. The results of clinical studies evaluating the activity of rituximab when used alone or in combination with other antileukemia agents for the treatment of this disease are reviewed here.
Similar content being viewed by others
References
Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456.
US Food and Drug Administration. FDA approves rituxan to treat chronic lymphocytic leukemia. Available at: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm201069.htm. Accessed May 18, 2011.
Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29(Suppl. 2):2–9.
Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368.
Robak T, Dmoszynska A, Solal-Celigny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1765.
Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. J Immunol. 2007;179:4263–4271.
Williams ME, Densmore JJ, Pawluczkowycz AW, et al. Thrice-weekly low-dose rituximab decreases CD20 loss via shaving and promotes enhanced targeting in chronic lymphocytic leukemia. J Immunol. 2006;177:7435–7443.
Aue G, Lindorfer MA, Beum PV, et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2010;95:329–332.
Berinstein NL, Grillo-Lopez AJ, White CA, Bence et al. Association of serum rituximab (IDECC2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9:995–1001.
Regazzi MB, Iacona I, Avanzini MA, et al. Pharmacokinetic behavior of rituximab: a study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit. 2005;27:785–792.
Maloney DG, Liles TM, Czerwinski DK, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–2466.
Tobinai K, Kobayashi Y, Narabayashi M, et al. Feasibility and pharmacokinetic study of a chimeric anti-CD20 monoclonal antibody (IDEC-C2B8, rituximab) in relapsed B-cell lymphoma. The IDEC-C2B8 Study Group. Ann Oncol. 1998;9:527–534.
Tobinai K, Igarashi T, Itoh K, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–830.
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol.1997;15:3266–3274.
McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833.
O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–2170.
Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751.
Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood. 2003;101:6–14.
Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood. 2005;105:49–53.
Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088.
Keating MJ, O’Brien S, Tam C, Lerner S, Kantarjian H. Salvage therapy following failure or relapse after FCR chemo-immunotherapy as initial treatment for chronic lymphocytic leukemia (CLL). J Clin Oncol. 2007;25(Suppl.18): Abstract 7009.
Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174.
Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood. 2007;109:405–411.
Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:498–503.
Lamanna N, Jurcic JG, Noy A, et al. Sequential therapy with fludarabine, high-dose cyclophosphamide, and rituximab in previously untreated patients with chronic lymphocytic leukemia produces high-quality responses: molecular remissions predict for durable complete responses. J Clin Oncol. 2009;27:491–497.
Bosch F, Abrisqueta P, Villamor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone: a new, highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4578–4584.
Faderl S, Wierda W, O’Brien S, Ferrajoli A, Lerner S, Keating MJ. Fludarabine, cyclophosphamide, mitoxantrone plus rituximab (FCM-R) in frontline CLL <70 years. Leuk Res. 2010;34:284–288.
Fischer K, Stilgenbauer S, Schweighofer CD, et al. Bendamustine in combination with rituximab (BR) for patients with relapsed chronic lymphocytic leukemia (CLL): a multicentre phase II trial of the German CLL Study Group (GCLLSG). American Society of Hematology Annual Meeting, December 6–9, 2008, San Fransisco, CA. Abstract 330.
Parikh SA, O’Brien S, Ferrajoli A. Frontline combined chemoimmunotherapy with fludarabine, cyclophosphamide, alemtuzumab and rituximab (CFAR) in high-risk chronic lymphocytic leukemia. American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 208.
Di Gaetano N, Xiao Y, Erba E, et al. Synergism between fludarabine and rituximab revealed in a follicular lymphoma cell line resistant to the cytotoxic activity of either drug alone. Br J Haematol. 2001;114:800–809.
Alas S, Bonavida B, Emmanouilides C. Potentiation of fludarabine cytotoxicity on non-Hodgkin’s lymphoma by pentoxifylline and rituximab. Anticancer Res. 2000;20:2961–2966.
Schulz H, Klein SK, Rehwald U, et al. Phase 2 study of a combined immunochemotherapy using rituximab and fludarabine in patients with chronic lymphocytic leukemia. Blood. 2002;100:3115–3120.
Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–2164.
Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol. 2006;24:437–443.
Flinn IW, Neuberg DS, Grever MR, et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol. 2007;25:793–798.
Eichhorst BF, Busch R, Hopfinger G, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood. 2006;107:885–891.
Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980.
Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113:3168–3171.
Constantine ST, Wierda GW, O’Brien S, et al. Life after fludarabine, cyclophosphamide, & rituximab (FCR) — the clinical outcome of patients with chronic lymphocytic leukemia who receive salvage treatment after frontline FCR. American Society of Hematology Annual Meeting, December 6–9, 2008, San Fransisco, CA. Abstract 2090.
Tam CS, Otero-Palacios J, Abruzzo LV, et al. Chronic lymphocytic leukaemia CD20 expression is dependent on the genetic subtype: a study of quantitative flow cytometry and fluorescent insitu hybridization in 510 patients. Br J Haematol. 2008;141:36–40.
Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916.
Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer. 2007;109:2291–2298.
Kay NE, Wu W, Kabat B, et al. Pentostatin and rituximab therapy for previously untreated patients with B-cell chronic lymphocytic leukemia. Cancer. 2010;116:2180–2187.
Reynolds CM, Di Bella N, Lyons RM, et al. Phase III trial of fludarabine, cyclophosphamide, and rituximab vs. pentostatin, cyclophosphamide, and rituximab in B-cell chronic lymphocytic leukemia. American Society of Hematology Annual Meeting, December 6–9, 2008, San Fransisco, CA. Abstract 327.
Fischer KPC, Stilgenbauer S, Busch R, et al. Bendamustine combined with rituximab (BR) in first-line therapy of advanced CLL: a multicenter phase II trial of the German CLL Study Group (GCLLSG). American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 205.
Carney DA, Westerman DA, Tam CS, et al. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062.
Wierda W, O’Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078.
Byrd JC, Kipps TJ, Flinn IW, et al. Phase 1/2 study of lumiliximab combined with fludarabine, cyclophosphamide, and rituximab in patients with relapsed or refractory chronic lymphocytic leukemia. Blood. 2010;115:489–495.
Lamanna N, Kalaycio M, Maslak P, et al. Pentostatin, cyclophosphamide, and rituximab is an active, well-tolerated regimen for patients with previously treated chronic lymphocytic leukemia. J Clin Oncol. 2006;24:1575–1581.
Bertazzoni P, Rabascio C, Gigli F, et al. Rituximab and subcutaneous cladribine in chronic lymphocytic leukemia for newly diagnosed and relapsed patients. Leuk Lymphoma. 2010;51:1485–1493.
Robak T, Smolewski P, Cebula B, Grzybowska-Izydorczyk O, Blonski JZ. Rituximab plus cladribine with or without cyclophosphamide in patients with relapsed or refractory chronic lymphocytic leukemia. Eur J Haematol. 2007;79:107–113.
Badoux Xavier C. MK, Susan O’Brien, et al. Chemoimmunotherapy with cyclophosphomide, fludarabine, alemtuzumab and rituximab (CFAR) is effective in relapsed patients with chronic lymphocytic leukemia (CLL). American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 3431.
Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203.
Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia. 2009;23:1779–1789.
Castro JE, Sandoval-Sus JD, Bole J, Rassenti L, Kipps TJ. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia. 2008;22:2048–2053.
Woyach JA, Lin TS, Lucas MS, et al. A phase I/II study of rituximab and etanercept in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia. 2009;23:912–918.
Zent CS, Call TG, Shanafelt TD, et al. Early treatment of high-risk chronic lymphocytic leukemia with alemtuzumab and rituximab. Cancer. 2008;113:2110–2118.
Faderl S, Ferrajoli A, Wierda W, O’Brien S, Lerner S, Keating MJ. Alemtuzumab by continuous intravenous infusion followed by subcutaneous injection plus rituximab in the treatment of patients with chronic lymphocytic leukemia recurrence. Cancer. 2010;116:2360–2365.
Faderl S, Thomas DA, O’Brien S, et al. Experience with alemtuzumab plus rituximab in patients with relapsed and refractory lymphoid malignancies. Blood. 2003;101:3413–3415.
Ferrajoli A. Incorporating the use of GM-CSF in the treatment of chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:514–516.
Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45.
James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the initial treatment of chronic lymphocytic leukemia: report of an ongoing study. J Clin Oncol. 2010;28(Suppl. 15). Abstract 6583.
Ferrajoli AXCB, O’Brien S, Wierda WG et al. Combination therapy with lenalidomide and rituximab in patients with relapsed chronic lymphocytic leukemia (CLL). American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 206.
Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297.
Del Poeta G, Del Principe MI, Consalvo MA, et al. The addition of rituximab to fludarabine improves clinical outcome in untreated patients with ZAP-70-negative chronic lymphocytic leukemia. Cancer. 2005;104:2743–2752.
Del Poeta G, Del Principe MI, Buccisano F, et al. Consolidation and maintenance immunotherapy with rituximab improve clinical outcome in patients with B-cell chronic lymphocytic leukemia. Cancer. 2008;112:119–128.
Carson KR, Evens AM, Richey EA, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840.
D’souza A, Wilson J, Mukherjee S, Jaiyesimi I. Progressive multifocal leukoencephalopathy in chronic lymphocytic leukemia: a report of three cases and review of the literature. Clin Lymphoma Myeloma Leukemia. 2010;10:E1–E9.
Niscola P, Del Principe MI, Maurillo L, et al. Fulminant B hepatitis in a surface antigen-negative patient with B-cell chronic lymphocytic leukaemia after rituximab therapy. Leukemia. 2005;19:1840–1841.
Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800.
Wierda WG, Kipps TJ, Dürig J, Griskevicius L, et al. Chemoimmunotherapy with ofatumumab, fludarabine, and cyclophosphamide (O-FC) in previously untreated patients with chronic lymphocytic leukemia (CLL). J Clin Oncol. 2010;28(Suppl. 15). Abstract 6520.
Patz M, Forcob N, Müller B, et al. Depletion of chronic lymphocytic leukemia cells from whole blood samples mediated by the anti-CD20 antibodies rituximab and GA101 [abstract]. American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 2365.
Morschhauser FGC, Lamy T, Milpied NJ, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia. American Society of Hematology Annual Meeting, December 5–8, 2009, New Orleans, LA. Abstract 884.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
James, D.F., Kipps, T.J. Rituximab in chronic lymphocytic leukemia. Adv Therapy 28, 534–554 (2011). https://doi.org/10.1007/s12325-011-0032-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-011-0032-2