Abstract
Beyond motor deficits, spinocerebellar ataxia (SCA) patients also suffer cognitive decline and show socio-affective difficulties, negatively impacting on their social functioning. The possibility to modulate cerebello-cerebral networks involved in social cognition through cerebellar neurostimulation has opened up potential therapeutic applications for ameliorating social and affective difficulties. The present review offers an overview of the research on cerebellar neurostimulation for the modulation of socio-affective functions in both healthy individuals and different clinical populations, published in the time period 2000–2022. A total of 25 records reporting either transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) studies were found. The investigated clinical populations comprised different pathological conditions, including but not limited to SCA syndromes. The reviewed evidence supports that cerebellar neurostimulation is effective in improving social abilities in healthy individuals and reducing social and affective symptoms in different neurological and psychiatric populations associated with cerebellar damage or with impairments in functions that involve the cerebellum. These findings encourage to further explore the rehabilitative effects of cerebellar neurostimulation on socio-affective deficits experienced by patients with cerebellar abnormalities, as SCA patients. Nevertheless, conclusions remain tentative at this stage due to the heterogeneity characterizing stimulation protocols, study methodologies and patients’ samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Cerebellar Role in Socio-Affective Functions
Over the last decades, a consensus has been reached about the role of the cerebellum in affective and social functions [1, 2] and increasing evidence has emerged about its inclusion in the cortico-limbic networks subserving emotion processing [1, 3, 4]. Indeed, emotional processing is considered one of the main components of social cognition [5], defined as a set of mental processes engaged by humans to comprehend, produce, and regulate social behavior to interact with others in a social environment [6, 7]. A fundamental aspect of social cognition is Theory of Mind (ToM), or the “mentalizing” process, i.e. the ability to attribute mental states (such as emotions, intentions, and beliefs) to others to explain and predict their behavior [8, 9].
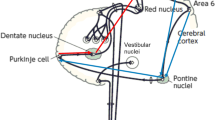
The cerebellar connectional and functional topography provides the critical anatomical substrate [10] to understand the functions of the cerebellum, including its role in social cognition. It is commonly assumed that the cerebellum operates as a co-processor of a wide range of functions, by modulating the activity of key cerebral regions to which different cerebellar modules are connected [11]. This cerebro-cerebellar connectivity thus affects sensory-motor processing as well as cognitive and affective functions [2, 10].
More in general, the sequence detection theory [12] suggests that the cerebellar operational model is the same regardless of whether the information to be processed is sensory-motor, cognitive, or behavioral. According to this model, the cerebellum detects and memorizes patterns by constructing internal models of the experienced sequence of events. This predictive and sequential coding can be also extended to social behavior [2, 13, 14], and emotion regulation [15]. The idea is that the cerebellum may modulate cerebral activity to promote the correct implementation of social action sequences, and to adjust unexpected events when violations from predicted scenarios are met [12].
According to the “dysmetria of thought” hypothesis by Schmahmann and colleagues [16], cerebellum-related affective and cognitive deficits would mirror diminished (hypometric) or exaggerated (hypermetric) responses to the internal and/or external environment [17]. In this view, cerebellar structural alterations may affect the modulatory function of the cerebellum on the cortical projection areas involved in emotional and social processing, so that behavior is not always appropriately adjusted to specific social environmental requirements [2, 14]. This interference may lead to specific impaired social outcomes, particularly when the specific situation/interaction requires advanced ToM abilities and a high level of prediction.
Social cognition and high-level ToM functions require complex interactions between limbic, associative, and subcortical areas [18,19,20,21]. ToM abilities seem to mainly depend on a group of brain regions, called the “mentalizing network,” which includes regions in the superior temporal sulcus (STS), temporoparietal junction (TPJ), medial precuneus, and dorso medial prefrontal cortex (dmPFC, [22, 23]).
It is widely acknowledged that the cerebellum is incorporated into associative and paralimbic circuits involved in affective and social processes [24, 25]. The cerebellar involvement in the social brain network has been widely supported by resting-state fMRI studies. Indeed, the investigation of functional connectivity has identified a cerebellar topography for social functions showing neural synchronization between distinct cerebellar and cerebral zones, known to be strictly related to affective functions and social mentalizing [26, 27]. Functional coherence has been found between the cerebellar vermis and brain limbic structures typically implicated in emotional regulation [28], such as the hippocampus, involved in memory and learning processes [29], and the amygdala, known to modulate distinct aspects of emotional processes [30]. The cerebello-cerebral network related to the most abstract and complex forms of mentalizing has been specifically characterized by a multi-study analysis of Van Overwalle and Mariën [21] and included the dmPFC, precuneus/ posterior cingulate cortex, bilateral TPJ, and a region in the posterior cerebellum corresponding to the right Crus II. Overall, these observations suggest that some areas of the cerebellum may be preferentially recruited for specific components of social mentalizing.
While many functional studies showed cerebellar activations during emotional processing and mental state inference tasks [31, 32], clinical studies provided further support to the view of a “social cerebellum” reporting an impaired performance of patients with cerebellar damage in a range of perceptual [33], affective, cognitive [34, 35], and ToM tasks [36] that are essential in social interactions. The prevalent idea is that these impairments may be caused by abnormal cerebellar modulation of cerebral areas involved in emotional and mentalizing processing, such as limbic, frontal, and temporo-parietal areas [21, 37, 38].
Social and Affective Disturbances in Hereditary Ataxia
Increasing evidence suggests the occurrence of emotional and social disturbances in patients with various types of cerebellar diseases, affecting the quality of their social life [14, 39, 40]. In the context of cerebellar disease, the spinocerebellar ataxias (SCAs) are a group of rare (prevalence rate of 1–4 in 100,000) [41] neurodegenerative disorders of autosomal dominant inheritance resulting from degeneration of the cerebellum and its connections. Many studies demonstrated the presence of cognitive [42, 43] emotional and neuropsychiatric disorders in these pathologies [35, 44] grouped in the so-called “cerebellar cognitive affective syndrome” (CCAS) [34].
Starting from these observations, an increasing body of studies has reported that SCA patients also present alterations in various aspects of social cognition, from the perception of emotions to ToM [45,46,47]. The results about the pattern of observed impairments, however, have been conflicting [48]. Some authors suggest that the cerebellum may be exclusively involved in more complex aspects of social cognition, showing an impairment of ToM abilities in patients with SCA3 and SCA6 [49], whereas the attribution of basic emotions evaluated by verbal tasks would be spared. However, other studies reported that SCA2 and SCA7 patients are impaired also in verbal emotion attribution tasks, thus suggesting that social cognition impairment in SCA patients is not homogeneous among the various genotypes [47]. Authors showed that patients with SCA2 and SCA7 present difficulties in attributing emotions, such as happiness, sadness, fear, anger, and embarrassment, corroborating previous findings of cerebellar involvement in emotional processing [47], (for a review see also [1]). In the study of Sokolovsky and colleagues [47], no emotional impairment was reported in SCA1 patients, while a ToM impairment was described in only one SCA1 patient, with no evidence of this deficit in any of the SCA2 or SCA7 patients. According to the described findings [47, 49], the distinct impairment profiles of the five patient groups (SCA1, SCA2, SCA3, SCA6, and SCA7) can be explained by the segregation of functions within the cerebellum.
A later study by D’Agata and colleagues (2011) showed that emotion recognition, as assessed by the Ekman 60 Faces battery and the Tamietto 50 Faces test [50, 51], is impaired in patients with hereditary ataxia of different genotypes, supporting the evidence previously reported in SCA2 and SCA7 and adding evidence of emotion recognition impairment in SCA6 and SCA8 patients. In particular, this study indicated that SCA patients have a prominent deficit in the identification of more complex social emotions, both positive and negative, with respect to basic emotions [45]. These findings are supported by another study using the revised Reading the Mind in the Eyes (RMET) [52] showing that both patients with complex cerebrocerebellar degeneration (i.e. SCA1, SCA2, SCA7, SCA17) and those with an isolated cerebellar disease (i.e. SCA3, SCA6, episodic ataxia type2) were impaired in emotion attribution and in processing negative and positive emotions compared to emotionally neutral stimuli [46]. These findings are in line with the evidence that the cerebellum participates in the complex network that processes emotional stimuli, especially those having an emotionally negative valence [53, 54].
In terms of underlying neural substrate, neuroimaging studies have supported the relationship between structural and functional cerebellar alterations and the impairment of different social cognition abilities in different cerebellar patients [13, 39, 40]. The gray matter (GM) reduction in specific portions of the cerebellum (vermis and bilateral Crus I/II) has been linked to social impairment in patients affected by cerebellar neurodegenerative pathologies [13]. Intriguingly, these areas showed decreased functional connectivity with cerebral areas involved in mirroring and mentalizing processing [20, 21].
Altered cerebello-cerebral functional coupling has been also related to social impairment in patients with hereditary ataxia. Aberrant inter-nodal functional connectivity between the posterior cerebellum and cerebral regions related to social cognition processing was recently found in a homogenous population of patients affected by SCA2 [40]. These results suggest that the atrophy of specific cerebellar portions and disruption of the cerebello-cortical pathway may subtend the social cognition deficits in SCA2. Consistently, a more recent study provided an extensive characterization of the social cognition profile of SCA2 patients, who showed impairment in the immediate perceptual component of the mental state recognition (i.e., recognizing feelings and thoughts of other people from eye expressions) and difficulties in understanding false or mistaken beliefs as assessed by the RMET [52] and Faux Pas [55] tests, respectively. Interestingly, the authors found that patients’ performance on each impaired task correlated with specific MRI changes. A direct correlation was found between alterations in more complex components of social mentalizing, as assessed by the Faux Pas, and GM volumes in the right Crus II [39].
Further evidence comes from another recent study showing that patients with hereditary (SCA1, SCA2) and idiopathic ataxia exhibit impaired performance on both the Faux Pas Recognition Test and the RMET [56]. Patients have difficulty in understanding the mental states of others in everyday interactions and from their facial expressions.
Overall, the present results suggest that social cognition presents both typical and specific alterations according to the SCA variant [57]. The characterization of social and emotional features in different SCA subtypes may help the management of patients’ quality of life and could serve as a possible preclinical marker of the disease. Most importantly, the present findings have opened a substantial body of studies investigating the modulating effects of cerebellar stimulation on social skills. This may have important implications in the clinical and translational field to consider the cerebellum as a potential neurostimulation target across multiple pathological conditions.
Non-Invasive Brain Stimulation
Non-Invasive Brain Stimulation (NIBS) techniques are widely used in healthy adults to investigate brain mechanisms or to modulate and enhance cognitive and socio-affective processes [58]. NIBS techniques, both transcranial magnetic stimulation (TMS) and different forms of transcranial electrical stimulation, including transcranial direct current stimulation (tDCS), are used to boost neuropsychological or psychiatric rehabilitation, through modulation of neuroplasticity. In TMS protocols, a coil placed above the scalp delivers a brief and high-amplitude current and generates a magnetic pulse that induces a transitory electric current in the cerebral surface under the coil. With sufficient intensity, a single pulse of TMS causes highly synchronized action potentials in the targeted area. TMS can be delivered as a single-pulse, repetitive (i.e., rTMS, series of pulse trains) or in a patterned fashion, such as theta-burst stimulation (TBS), in which a series of pulses are delivered in bursts of high frequency (i.e., 50 Hz) with an interburst interval of 200 ms (i.e., 5 Hz). TMS represents a powerful tool for investigating causal brain-behavior relations complementing correlative techniques such as functional neuroimaging. Indeed, if stimulating a cortical region significantly affects task performance, this indicates that the targeted area is necessary to perform the task normally. Therefore, TMS effects have traditionally been interpreted to interfere with brain function, by inducing a transient, reversible “virtual lesion” in the targeted region, with impairment as its default outcome [59, 60]. TMS could also enhance brain activity and behavioral performance (for a review, [61]). Indeed, TMS behavioral effects are state-dependent, and factors such as stimulation parameters, task difficulty, and cognitive state can fundamentally change the stimulation outcome [62,63,64]. For instance, rTMS-induced effects are frequency-dependent, with low (≤ 1 Hz) and high frequencies (≥ 5 Hz) decreasing and increasing cortical excitability, respectively (for a review, [65]. As for TBS, continuous TBS (i.e., cTBS), in which bursts of pulses are delivered without interruption, reduces cortical excitability, while intermittent TBS (i.e., iTBS), in which short intervals separate bursts of pulses, enhances it. TMS has a relatively good spatial resolution, (see [66] for more details) and a high temporal resolution (see [67, 68] for examples). TMS can be applied using either an online or an offline protocol. In online protocols, either single pulses or short trains of pulses (typically delivered at 10 or 20 Hz) are delivered while individuals are engaged in a task (for review, [69]). In offline paradigms, task performance is assessed before and after the stimulation, during which a series of pulse trains are applied over a period typically lasting 10 to 20 min, with stimulation aftereffects on behavioral performance outlasting the period of stimulation by many minutes or hours (depending on the stimulation protocol and its parameters).
In tDCS protocols, the current is typically delivered using a bipolar montage consisting of the active electrode (anode or cathode, depending on the experimental design) located directly over the targeted region and the reference electrode located over either a cephalic site (commonly, the contralateral supraorbital region) or an extracephalic site (e.g., the deltoid or the buccinators muscles). tDCS does not directly induce cerebral activity, but it rather alters spontaneous brain excitability by subthreshold modulation of the neural resting state potential [70]. Currently, tDCS devices apply a weak direct electrical current (0.5–2 mA), typically for a relatively long period of time (e.g., 20 min). Depending on the electrode polarity, the stimulation facilitates (anodal) or inhibits (cathodal) spontaneous neuronal activity resulting in modulation of neuronal excitability and neuroplastic reorganization [70]. However, as for TMS, physiological and behavioral tDCS-induced effects depend on a complex interaction between stimulation parameters and endogenous neural activity (e.g., [71, 72]). This aspect is particularly relevant when stimulating the cerebellum. Indeed, given its entirely different cytoarchitecture compared to the neocortex, the cerebellar cell morphology, and the complex cerebellar folding, when applied to the cerebellum, tDCS polarity is not predictive of the direction of the behavioral changes (for a meta-analysis see [73]). Similarly to TMS, tDCS can be administered either online or offline (see [74]). Ten minutes or more of tDCS can lead to modulatory effects that outlast the period of stimulation by many minutes or hours, with more robust behavioral effects being detectable immediately after the end of the stimulation (for reviews, [75]).
Aim of the Present Scoping Review
This scoping review aims to present an overview of the research on cerebellar neurostimulation to modulate socio-affective functions in the healthy and clinical population. We decided to include in our search also studies employing NIBS in patients with other clinical pathologies, beyond SCA syndromes, in line with evidence reporting cerebellar structural and functional abnormalities in a large variety of neurological and neuropsychiatric conditions [76]. Indeed, the existence of cortico-cerebellar and cerebellar limbic networks involved in social cognition makes the cerebellum a promising target candidate to modulate socio-affective functions. Therefore, notwithstanding the differences in etiology-related factors, the effects on socio-affective functions achieved via cerebellar NIBS in other pathologies associated with cerebellar anomalies may generalize to the SCA population. Hence, this scoping review aims to foster a discussion on potential therapeutic approaches to treat the socio-affective symptoms afflicting SCA patients. We decided to perform a scoping review and not a systematic review due to the heterogeneity of the literature on the topic. This heterogeneity can be detected in the stimulation tools, in the outcome measures, in the clinical conditions as well as in the scopes. Indeed, the included studies aimed either at investigating the contribution of the cerebellum to specific socio-affective functions using neurostimulation tools or at examining the effectiveness of these tools targeting the cerebellum in reducing socio-affective symptoms or improving social functioning in clinical populations with cerebellar damage or with impairments in functions that involve the cerebellum. This review focused on articles published between January 2000 and December 2022 and included exclusively primary research studies. To our knowledge, no reviews on the same issue are available in the extant literature. Even though SCA syndromes represent a relatively rare condition with a low prevalence rate in the population, the costs and burdens associated with the disease, in all its clinical manifestations, have a significant impact on the quality of life of these patients and their caregivers [77]. Hence, reviewing the literature on this topic should be considered a matter of interest not only for research scopes but also for clinical practice.
Materials and Methods
This review was conducted according to the framework proposed by Peters et al. [78] for scoping studies.
Identifying the Review Questions
The research question was “What is the evidence, described in the published literature, of cerebellar neuro-stimulation boosting effects on socio-affective functions in the clinical and healthy population?”. The aims of the present review were to i) map and summarize the evidence on the boosting effects of cerebellar neuro-stimulation on socio-affective functions, and ii) develop a discussion on potential rehabilitation implications for hereditary ataxia patients.
Inclusion Criteria
Criteria for study inclusion were the following: i) usage of NIBS techniques targeting the cerebellum in humans for ii) the modulation of socio-affective functions, as assessed by performance-based measures, questionnaires or qualitative outcomes (e.g., verbal report), reported in iii) research articles published in peer-reviewed English-language journals. Records considered as not pertinent were the following: animal studies, studies using deep brain stimulation techniques, or other stimulation tools (e.g., vagus nerve stimulation), applying NIBS targeting other brain areas or applying cerebellar NIBS but measuring exclusively non-social outcomes (i.e., other cognitive or motor functions or physiological measures), neuroimaging studies or studies applying any other investigation techniques but NIBS (e.g., pharmacological investigations), as well as any document other than primary research articles (e.g., review, book chapter, commentary etc.). At each selection process step, if one or more of these exclusion criteria were found in screening the record – thus, if information indicative of the presence of any exclusion criteria was detected in the title, abstract or text – then the record was not selected for inclusion. Studies applying NIBS in humans and examining both social and non-social outcomes were included, but only the information regarding the social outcomes was extracted and summarized in the following steps. We considered “socio-affective” a broad range of measures assessing social skills (e.g., actions comprehension or biological motion discrimination), mental health problems (e.g., depressive and/or anxiety symptoms severity), affective states or emotion regulation abilities.
Search Strategy
The literature search was conducted by two authors, V.O and A.C., by screening scientific online databases (PubMed, Scopus and Web of Science) to identify pertinent studies, using the following text string: (“brain stimulation” OR neuromodulation OR “transcranial direct current stimulation” OR “transcranial magnetic stimulation” OR tES) AND (cerebellum OR cerebellar) AND (social OR emotion OR affective OR mood). The period considered for study inclusion was January 2000-December 2022. No other restrictions on document type or language of records were posed.
Evidence Screening and Selection
After the removal of duplicates, V.O. and A.C. screened the titles of the records, (title screening) identified by the search string and excluded those records not fitting with the topic of the review. Of the remaining records, they screened the abstract (abstract screening) and excluded those whose content was judged not to be relevant for the present review. In case some methodological information could not be retrieved by screening the abstracts, the two authors read the full texts (text screening) to determine whether to include the record. The list of records, after duplicates removal, was divided into two parts; one reviewer screened the first half, the other the second half. In case of disagreement in records selection for inclusion, other two authors, C.U. and Z.C., were consulted to discuss the reasons for the disagreement and to deliberate upon study inclusion. Figure 1 depicts the study selection procedure.
Data Extraction and Charting
Data were charted referring to the review question “What is the evidence, described in the published literature, of cerebellar neuro-stimulation boosting effects on socio-affective functions in the clinical and healthy population?”.
The list of records selected for inclusion was divided into two parts. For each part, data were extracted by one reviewer and a 25% sample was checked for accuracy and completeness by the other [79].
Studies were categorized according to the neuro-stimulation technique applied, namely TMS or tDCS, and the target population, namely healthy volunteers or clinical samples. TMS and tDCS studies were schematized in separate tables, as the two techniques present different technical characteristics and modalities of usage. First, we reviewed TMS and then tDCS studies.
The table reporting the technical details of the TMS protocols (Table 1) summarizes the following information: authors and year of publication; type of stimulation protocol (e.g., repetitive or single-pulse); target site(s); type of coil used; frequency and intensity of the stimulation; number of sessions; timing of the stimulation (online or offline); whether or not an MRI-guided navigation system was used; information on whether TMS-induced sensations were reported or not. The table reporting the technical details of the tDCS protocols (Table 2) summarizes the following information: first author and year of publication; montage of electrodes; electrodes size; stimulation intensity; number of sessions; timing of the stimulation (online or offline); information on whether tDCS-induced sensations were reported or not.
The tables on the methodological aspects, for both TMS (Table 3) and tDCS (Table 4) studies, report this information: first author and year of publication; research design (specifying the within- and between-participants factors); whether the study was sham-controlled; applied a blinding procedure; conducted a power-analysis for the estimation of the sample size; reported the effect size(s); and included a follow-up to detect long-lasting effects of the stimulation; sample size and characteristics (health status or diagnosis, age, sex); whether or not medication intake and any (other) medical conditions of the participants were reported or controlled for; outcome measures and details of these (e.g., type of task); reference socio-affective domain (classified as “social” or “affective”). In this regard, the outcomes referring to mental health problems, symptoms severity, emotion regulation abilities, as well as changes in mood and affective states, were labeled as “affective”. The outcomes referring to the cognitive mechanisms involved in the processing of social stimuli (e.g., emotion or action processing) were labeled as “social”. Lastly, it was indicated whether each study reported a significant modulation effect on at least one socio-affective outcome from all those assessed.
Analysis of the Evidence and Presentation of Results
The first section describes the results of the research strategy and selection process. In the second section, data were synthesized in a descriptive format, in separate paragraphs and tables according to the specific neuro-stimulation tool applied in each study, to identify different aspects of the literature as outlined in the key question. The occurrence of stimulation technical details, methodological choices and outcome characteristics were reported.
Results
Study Selection for Review
The literature search identified a total of 571 records, 126 from Pubmed, 265 from Scopus and 180 from Web of Science. After the removal of duplicates (n = 190), the titles of 381 records were screened. Of these, 117 records were excluded as non-pertinent whenever the title was indicative of the presence of one or more of the exclusion criteria described above. The abstracts of the remaining records (n = 264) were screened against the inclusion/exclusion criteria. This stage of the selection process led to the identification of 34 potentially eligible studies. After reading the text of these records, 9 were further excluded. Thus, 25 records were considered eligible and included in data extraction and charting.
TMS Protocols
Out of the 25 records included in the data extraction and charting phase, 14 (56%) applied the TMS. Of these, 9 (64.3% of the TMS studies) tested healthy participants, whereas 5 (35.7%) enrolled different clinical populations. Three studies (21.4%) applied a low frequency (1 Hz) repeated TMS protocol, 5 applied a theta-burst stimulation protocol, with frequencies ranging from 5 to 7 Hz, and the remaining 6 studies (42.9%) opted for a high frequency protocol, at 20 or 25 Hz. In one of the latter studies, the authors included an experiment delivering single-pulse TMS. The medial portion of the cerebellum was chosen as the main target site in 10 studies (71.4%). In 2 of these, TMS was also delivered to the left cerebellar hemisphere, and one of the 2 also targeted the right cerebellar hemisphere. Of the remaining studies, 3 targeted only the left cerebellar hemisphere and only one the right cerebellar hemisphere, besides other target non-cerebellar areas. In studies stimulating other cerebral areas (50%), the occipital cortex was chosen as a control site in 6 cases—given its proximity to the cerebellum and to rule out the possibility that any observed effect may depend on indirect stimulation of the visual cortex [103]—and the vertex in 3 cases.
As for the intensity parameter, 5 studies (35.7%) stimulated at 80% of the participants’ motor threshold (MT), 9 at 100%.
For what concerns the coil type and geometry, half of the studies used a figure-of-eight coil—likely stimulating more superficial, posterior regions of the cerebellum—4 studies used a double cone coil—recommended for effective cerebellar stimulation [104]—and 3 an iron-core coil.
As emerged for tDCS, clinical studies included a greater number of TMS sessions (n = 10) than studies on healthy volunteers. Only in De Vidovich et al. [91], which consists of a one-shot experiment, the clinical sample underwent a single TMS session. In 4 studies, all applying a triple-pulse paradigm, the TMS was delivered online, thus during stimuli presentation, whereas the remaining 10 studies adopted an offline paradigm. Although neuronavigated-TMS on individual magnetic resonance images (MRI) scans is encouraged to enable precise targeting and decrease interindividual variability [105], only 3 studies complied with this requirement. Other 4 studies, conducted by the same research group, localized the target areas employing stereotaxic navigation on individualized MRI scans, which were obtained through a 3D warping procedure fitting a high-resolution MRI template with the participant's scalp model and craniometric points. In the remaining 7 studies, the coil positioning was based on anatomical landmarks. In contrast with tDCS studies, half of the TMS studies did not report any information on cerebellar TMS tolerability and correlated sensations, including but not limited to pain and discomfort due to the contraction of neck muscles, often associated with this type of stimulation. Only 4 studies reported some degree of information on minor side effects (e.g., headache or sleepiness). Three studies did not describe any side effect or subjective sensation but commented that the stimulation was well tolerated or that information on tolerability was gathered by the experimenter.
TMS Studies: Methods and Characteristics
This paragraph provides a descriptive overview of the methodological features—schematized in Table 3—of the TMS studies.
Out of the 14 TMS studies, only 3 (21.4%) studies adopted a between-participant design, in which participants received either real or sham stimulation. The remaining 11 (78.6%) studies adopted a within-participant design, with all the participants receiving real TMS over the cerebellum, selected as the main target area, and a sham stimulation (in 2 cases) or real stimulation in other control target areas (in 6 cases). Three studies did not include any control condition (nor sham or active control sites). However, among these, the study by De Vidovich and colleagues (2016) aimed at comparing the performance of a group of patients with the performance of a group of healthy controls.
Overall, 5 (35.7%) studies were sham-controlled. The sham condition was obtained with either one of the following methods: using a modified coil, able to mimicking the sound click and sensation of real TMS, in which a metal plate was built in the housing directly under the iron-core (in 2 cases); setting the stimulation to a low intensity (10% of the individual MT); tilting the coil of 45 degrees; or applying shamming surface electrodes at the participants’ neckline, to simulate the tactile effects of the stimulation.
As for the blinding procedure, less than half of the studies (28.6%) adopted a double-blind procedure, while 5 studies adopted a single-blind procedure. No indication of the use of any blinding method was found in the remaining 5 studies. Surprisingly, only one study reported to have conducted a power analysis for the estimation of the sample size. In all other studies, no evidence of this analysis was found. For what concerns the inclusion of follow-up measures, they were detected only in 3 studies, all examining cerebellar TMS potential therapeutic effects in patients.
In total, the TMS studies included in the review process tested 445 participants, of which 367 healthy volunteers and 78 patients. All participants were adults. Except for the study of De Vidovich and colleagues (2016), which consisted of a one-shot experiment comparing the performance in a task of a group of patients with borderline personality disorder with that of a group of healthy controls, all 4 clinical studies enrolled patients diagnosed with schizophrenia and schizoaffective disorder. Cerebellar abnormalities have been observed in both borderline personality disorder and schizophrenia [106, 107].
The use of medications by the patients was either reported or controlled for in all clinical studies, including the sub-sample of patients tested by De Vidovich et al., [91]. For what concerns the studies testing healthy volunteers, only 3 studies failed to report this information. Evidence of screening of the participants’ health status was found in all studies. For what concerns the outcome measures, the review process depicts a heterogeneous picture of socio-affective functions, types of tasks and paradigms. The measures whose domain was labeled as “social” were all performance-based and included: explicit emotion processing tasks from facial expressions or body postures; implicit emotion processing tasks (e.g., during a color naming task or a gender recognition task); a Go/No Go task using words of either positive or negative valence; an attitude priming task requiring to categorize the valence of a series of adjectives primed by either an in-group or an out-group face; a picture and story sequencing task requiring to order sequences of actions involving true and false belief stories; and a biological emotion discrimination task. All these tasks were administered to healthy volunteers. Only 4 studies on healthy volunteers aimed at evaluating changes in mood and affective states. More precisely, in the study by Schutter and colleagues (2003) the elevation in mood observed after cerebellar TMS was not an outcome measure the authors had planned to monitor, but rather it was spontaneously reported by all the participants [109]. Affective measures administered to healthy volunteers included: an emotion regulation task and an emotion rating task following the exposition to emotion-eliciting pictures; standardized questionnaires for the evaluation of mood; VAS for the evaluation of changes in mood and affective states. All clinical studies assessed affective outcomes, including measures of affective states and symptoms severity, favoring standardized questionnaires.
With regards to the findings, 8 (57.1%) studies, including all the clinical studies, found that cerebellar TMS significantly improved at least one of the socio-affective outcomes among the assessed ones. It has to be noted that all the studies testing healthy volunteers reported a significant modulation effect of the stimulation, although in 6 cases (42.9%) these effects were not found to boost the performance but provided evidence on the involvement of the cerebellum in the socio-affective function examined. Only the one-shot experiment conducted by De Vidovich and colleagues (2016) did not find any significant effect in the healthy control group, whereas a positive effect of the stimulation was found in the group of patients. This could pave the way for a discussion of publication bias. The only study on healthy participants in which no effects were reported was that of De Vidovich et al. [91], but these negative results were published because matched with positive findings in patients. One may suspect that other studies with all negative findings could not be published because they are less likely to be accepted by journals [108]. That said, with the exception of the study by De Vidovich et al. [91], significant cerebellar stimulation effects on socio-affective functions were found in all the TMS studies included in the review process.
tDCS Protocols
Out of the 25 records included in the data extraction and charting phase, 11 (44%) were tDCS studies. Of these, 6 (54.5% of the tDCS studies) tested healthy participants, whereas 5 (45.5%) enrolled different clinical populations. The majority of the studies (81.8%) targeted the medial cerebellum, while only 2 targeted the right cerebellar hemisphere. Excluding intra-cephalic montages, the right deltoid muscle was chosen as the reference region in 6 studies. Only one study applied the reference electrode over the right buccinator, and only one study targeted the spinal lumbar enlargement. In the healthy sample studies, the cerebellum was targeted by both the anode and cathode electrodes in a between-participants design (4 studies), where participants received either anodal or cathodal cerebellar stimulation, or in a within-participant design (2 studies), where all participants underwent both stimulation types in distinct time points. The cerebellum received active anodal stimulation in 3 studies and active cathodal stimulation in the remaining 2 studies on patients. The intensity was set at 2 mA in the majority of the studies (81.8%), while in one case it was set at 1.5 mA. The only study on children applied an intensity of 1 mA, in accordance with the recommendations on the safety and tolerability of tDCS in the pediatric population [109]. The stimulation lasted 20 min in 9 studies and in no case it exceeded this amount of time, remaining within the recommended limits of safety [110]. It is not surprising that clinical studies involved a greater number of stimulation sessions, ranging from 5 sessions (in one study) to 20 sessions (in 3 studies), likely to maximize the potentially beneficial effects of the stimulation over time. As for the timing, only 3 studies (27.3%) examined the effect of the stimulation on the outcome online, namely during the delivery of the current, whereas in all other cases the effects were assessed at the end of the stimulation (offline). In conformity with the guidelines [110], 81.8% of the studies reported that information on tDCS tolerability was gathered from participants. Yet, only four studies described the specific tDCS-induced sensations (Gheorge et al., 2021,[93, 98]. Tingling, skin redness and trouble in concentrating (or dizziness) were reported in all three studies,itching, burning sensation, headache and sleepiness were reported in two of the studies; scalp pain was reported only in one study, as were nausea, fatigue and mood change. One study compared the extent of all the above-mentioned sensations during real and sham stimulation conditions values were very low for all measures and they were comparable between the two stimulation conditions, except that itching and skin redness were rated as greater for real (anodal) than sham stimulation [97]. Finally, only in 2 studies no information on tolerability was detected during data extraction.
tDCS Studies: Methods and Characteristics
This paragraph provides a descriptive overview of the methodological features of the tDCS studies, schematized in Table 4.
Out of the 11 tDCS studies, 6 (54.5%) investigated polarity-dependent effects (i.e., anodal vs. cathodal stimulation); in 2 cases in a within-participants design and in 4 cases in a between-participants design. None of these were clinical studies, in which either anodal or cathodal cerebellar effects were investigated. The majority of the studies (81.8%) were sham-controlled. Newstead and colleagues [93] conducted a sham-controlled experiment (Exp 1) and an experiment (Exp 2) in which tDCS effects were examined over time with no control condition. Of those studies (18.2%) not involving a sham-control condition, one compared a fronto-occipital montage to a fronto-cerebellar montage and the other one simply examined tDCS effects over time with no control condition. Less than half of the studies (36.4%) adopted a double-blind procedure, even though its adoption is recommended to minimize the potential effects of research bias when collecting data [111]. Five studies (45.5%) adopted a single-blind procedure, in which only participants, but not experimenters, were blind to the type of stimulation delivered. Only in 2 studies no information on the blinding procedure was detected during data extraction. As for the power analysis calculation for estimating the sample size, a benefit of conducting this analysis is that it helps researchers to maximize the probability of observing the expected (significant) effect in the smallest sample size suitable for the purpose [112]. However, only 4 studies reported a power analysis for sample size estimation. In one further case, the sample size was estimated based on the sample size reported in similar research. In 6 cases, no information on power analysis was reported.
There is consensus that authors should report not only indexes of statistical significance, examining whether the findings are likely to be due to chance, but also effect sizes, which help the reader to understand the magnitude of the observed differences found between conditions (or groups, treatments etc.) [113]. Nevertheless, only 5 studies complied with this recommendation. For what concerns the inclusion of follow-up measures, 4 studies, of which 3 on patients, monitored potential long-lasting effects of the stimulation after the last stimulation session. Five studies on healthy volunteers and 2 on patients consisted of one-shot experiments and, thus, did not plan any follow-up phase.
In total, the tDCS studies included in the review process tested 422 participants, of which 311 healthy volunteers and 111 patients. Only one study [100] recruited children. Among the clinical studies, 2 aimed at examining potential therapeutic effects of cerebellar tDCS on patients diagnosed with neurodegenerative ataxia of different etiology. The other 3 studies focused on the following pathologies of the nervous system: major depressive disorder, autism spectrum disorder (in children) and idiopathic Parkinson's disease. All these disorders have been reported to display structural and functional anomalies of the cerebellum [114,115,116].
For both clinical and healthy sample studies, information was reported on whether medication intake and any medical condition of the participants (besides the main diagnosis for which patients were enrolled in the first place) were reported or controlled for. Only 3 (27.3%) studies, all on healthy participants, did not report any information on medication intake, while the remaining 8 studies either reported which medications the participants were taking at the time of the study or stated that participants were screened for the use of medications. Only in one study, no evidence of screening of the participants’ health status was found, whereas in all the other studies (90.9%) the presence of any medical conditions was either reported or controlled for.
Similarly to what was observed reviewing the TMS studies, tDCS studies presented a high heterogeneity in the socio-affective outcomes, type of tasks and paradigms. Most studies (81.8%) measured exclusively (in 4 cases) or also (in 5 cases) “affective” outcome, where this term refers to measures assessing mental health problems, symptoms severity, emotion regulation abilities or changes in mood and affective states. A visual analog scale (VAS) was used to evaluate changes in mood and affective states in 6 studies. Four out of 5 studies examining patients used standardized questionnaires for the evaluation of symptom severity and psychological adjustment. The measures labeled as “social” were all performance-based and included measures of the ability to recognize emotion from pictures of faces (facial emotion recognition task) or of the ability to infer mental states from pictures of eyes (Read the Mind in the Eye Test, RMET). Another task required participants to form predictions of social actions, then tested in conditions of perceptual uncertainty, based on the probability of co-occurrence between a particular action and contextual elements (social actions prediction task). Lastly, it was labeled “social” a task that required participants to learn a sequence that included information about others’ beliefs, which might converge with or differ from reality, resulting in true and false beliefs respectively (Implicit Belief Serial Reaction Time task).
Overall, 7 (63.6%) studies found that cerebellar tDCS significantly improved at least one of the socio-affective outcomes among all those assessed. On the other hand, 4 studies did not find any significant effect (Table 4). Among these, Benussi and colleagues (2021) reported a null effect of the stimulation on the Cerebellar Cognitive Affective Syndrome Scale (CCAS) total score, which provides a global indication of both the cognitive and affective skills of the patient. However, they did not differentiate the affective from the cognitive sub-score. Hence, no definitive conclusion on the potential effect of cerebellar tDCS on the affective components as measured by this scale could be drawn in the context of this study.
Discussion
The cerebellum plays a crucial role in socio-affective functions [1, 2], likely mediating predictive mechanisms through the generation and learning of social action sequences [12]. Indeed, patients suffering from cerebellar alterations, such as hereditary ataxia, often show difficulties in recognizing and inferring others’ mental states [13, 39, 45, 47] and in regulating their own emotional state. Nevertheless, the available rehabilitation protocols for these conditions mainly focus on sensory-motor symptoms, while paying little attention to socio-affective deficits. Among non-pharmacological approaches, treatments applying NIBS (alone or in combination with other conventional interventions) seem to be effective for rehabilitation purposes on cognitive and socio-affective functions in several clinical conditions (e.g., [117, 118]. Here, we identified and summarized the evidence on the boosting effects of cerebellar neurostimulation on socio-affective functions to discuss potential rehabilitative implications for hereditary ataxia patients.
Cerebellar Neurostimulation Boosting Socio-Affective Functions in Healthy Individuals
In most of the reviewed studies, the stimulation of the posterior cerebellum (with both TMS and tDCS) was effective in modulating healthy participants’ abilities in processing others’ mental states, from low-level motor intentions to emotions and higher-level mental states (e.g., beliefs and attitudes). In particular, tDCS applied over the medial cerebellum improved the recognition of others’ emotional states as expressed by facial expressions [54] and pictures of the eye region [96]. Furthermore, high-frequency rTMS over the same region enhanced emotional facial expressions recognition, even when emotional expressions were irrelevant to the task at play (i.e., implicit) [82]. Similarly, triple-pulse TMS over the paravermal cerebellum (in particular Crus I/Crus II) affected the explicit and implicit processing of happy and angry facial expressions [85]. Important indications for the possible explanation of why cerebellar stimulation improves emotion recognition come from empirical evidence suggesting that the posterior cerebellum may be selectively involved in the processing of negative emotional signals. Indeed, Ferrucci and colleagues [54] observed a selective improvement in the processing of negative emotional facial expressions following both anodal and cathodal tDCS over the medial cerebellum. Similarly, triple-pulse TMS over the paravermal cerebellum affected the discrimination of negative body expressions (i.e., anger or sadness), leaving the discrimination of positive body expressions (i.e., happiness and surprise) unchanged [53]. These findings support prior neuroimaging evidence reporting selective cerebellar activations in response to negative emotional cues [119, 120]. The selective valence-related role of the posterior cerebellum may depend on its role in predictive mechanisms. Within this framework, an agent expressing a negative emotion (e.g., anger or fear) may signal a potential danger to the perceiver and trigger (motor) “fight or flight” reactions (e.g., [121]. Cerebellar neurostimulation may thus potentiate the preparatory mechanisms implemented by the cerebellum that may help to respond to a potential threat.
Direct evidence on the role of the cerebellum in predictive mechanisms in social cognition comes from the study by Oldrati et al. [95] in which participants had to predict an agent’s action intention based on available contextual information. Anodal tDCS over the medial cerebellum improved the ability to infer and predict others’ action intentions when these were embedded in moderately informative contexts, while cathodal stimulation hindered participants’ sensitivity in predicting actions only when presented in strongly (but not moderately) informative contexts. Critically, tDCS did not affect a non-social control task requiring participants to predict the movements of physical shapes. This finding seems to suggest a specific and beneficial effect of cerebellar stimulation (at least of its medial part) in forming expectations related to social events. In particular, the cerebellum would play a crucial role in context-based prediction, where the available context (e.g., a particular place/situation/person or objects available in a scene) activates stored mental models of what can be expected in similar contexts [122]. This allows the prediction of others’ actions, emotions, or mental states and the control of ongoing inter-actions necessary for successful social interactions. Further support for the role of the cerebellum as a predictive device acting based on contextual information comes from a TMS study testing the neural correlates of stereotypical associations, which are implicit social associations that are prevalent in a specific social context/culture [84]. In this study, triple-pulse TMS over the (right) posterior cerebellum between the presentation of an in-group or out-group face and a trait adjective that participants had to evaluate, affected the stereotypical association between positive traits and in-group members, thus suggesting that the posterior cerebellum processes social signals depending on the associated/learned social context. Within the predictive framework, the role of the cerebellum would be to identify and predict sequences of a person’s (social) actions by supporting the explicit or implicit learning of frequently executed sequences of actions and mental states [14, 123, 124]. Accordingly, Heleven et al. [86] showed that low-frequency rTMS over the medial cerebellum significantly improved healthy participants’ performance in a Picture and Story sequencing task, which involved the explicit generation of the correct chronological sequence of social and non-social stories. Crucially, no difference was observed between false belief and mechanical (non-social) control stories, suggesting a cerebellar domain-general role in sequence generation. Similarly, anodal tDCS over the medial cerebellum improved the ability to implicitly learn non-social sequences [97].
As for affect regulation, the reviewed studies provide interesting data, though with some less consistent findings. Following 20 min of high-frequency rTMS over the medial cerebellum, participants spontaneously reported elevations in alertness and elevated mood [80], whereas low-frequency TMS impaired participants’ emotion regulation abilities as measured through self-compiled scales [81]. Mood elevation has been also observed following the simultaneous stimulation of the right cerebellar hemisphere and left dorsolateral prefrontal cortex, with accumulative and potentiated effects following successive stimulations [93]. However, it is difficult to disentangle the selective effect of cerebellar stimulation from the well-established effects of stimulation of the left prefrontal cortex on mood regulation [125]. Affect regulation might be seen as part of a body energy regulation process that aims to maintain (body) energy balance (i.e., homeostasis) by predicting the body’s needs and preparing to meet them [126]. Thus, the role of the cerebellum in predictive mechanisms might explain also its involvement in the regulation of one’s affective states. Nevertheless, it is important to note that several studies did not observe any beneficial effect on mood or affect regulation following either anodal or cathodal tDCS [54, 94, 96], high-frequency TMS [82] or iTBS [83] targeting the cerebellum, an issue that requires further investigation.
Cerebellar Neurostimulation Boosting Socio-Affective Functions in Clinical Populations
Among the reviewed articles, only two applied cerebellar NIBS on hereditary ataxia patients in randomized, double-blind, sham-controlled trials [99, 101]. In Benussi et al., [99], repeated sessions of anodal tDCS using a cerebello-spinal montage (i.e., one electrode over the medial cerebellum and the other over the spinal lumbar enlargement) improved neurodegenerative ataxia patients’ motor abilities, cognition, and quality of life for weeks after the treatment with additive effects after two repeated treatments. Note that socio-affective functions were not addressed with specific outcome measures in this study, but they were measured only as part of broader scales or questionnaires assessing also cognitive abilities or more general quality of life aspects. Maas et al. [101], though, observed no effect on motor, cognitive and patient-reported outcomes evaluating depressive symptoms and mood states in a cohort of patients with a specific type of hereditary ataxia, SCA3 (not included in [99]) following repeated sessions of anodal stimulation over the medial cerebellum (with reference electrode over the right deltoid muscle). Hence, given the differences in patients’ diagnosis, electrode montages, and outcome measures, it is premature to draw conclusions about the effectiveness of cerebellar neurostimulation in enhancing hereditary ataxia patients' socio-affective functions based on this evidence and further systematic investigations are necessary.
Nevertheless, studies employing cerebellar stimulation in other neurological and psychiatric conditions that are associated with cerebellar alterations may offer promising insight towards the implementation of innovative treatment protocols for hereditary ataxia patients also in the socio-affective domain. For instance, the ability to make accurate predictions in both social and non-social domains is impacted in autism spectrum disorder (ASD) patients [127, 128], who also show impaired ability to make predictions about their internal state [129], likely due to cerebellar structural and functional alterations (for review see [130, 131]). Among the reviewed studies, only one targeted the cerebellum in a small cohort of children with ASD [100], and showed a general reduction in symptoms global severity, particularly those related to social withdrawal and lethargy, hyperactivity, and mood, following 20 daily sessions of cathodal stimulation of the right cerebellar hemisphere and anodal over the left dorsolateral prefrontal cortex. Several studies among those considered here applied cerebellar NIBS to boost socio-affective functions in schizophrenia, a psychiatric condition in which imprecise predictive coding may represent a core pathological factor [106, 132], with symptoms’ severity being associated with reduced cerebellar grey matter volume and altered resting-state functional connectivity [133,134,135]. In line with this, a reduction of negative symptoms, including blunted affect, decreased motivation, social withdrawal, and anhedonia (as assessed by clinicians) has been observed following repeated sessions of iTBS over the medial cerebellum [88,89,90, 92]. In addition, patients reported positive effects on mood and depressive symptoms [88,89,90], but see [92]. Furthermore, patients with mood disorders may also present cerebellar gray matter loss and altered cerebellar-prefrontal connectivity [3, 114, 136]. Crucially, these difficulties in emotion regulation have also been associated with inefficient predictive coding [137] that leads to uncertainty and chronically elevated levels of distress and negative mood [126]. Accordingly, four weeks of simultaneous cathodal cerebellar stimulation and anodal left prefrontal cortex stimulation resulted in beneficial modulations of mood and depressive symptoms in patients with Major Depression, although with a weaker effect compared to a fronto-occipital montage [98].
Interestingly, cerebellar neurostimulation has been proven to be effective in modulating social and affective functions of patients with neurological and psychiatric conditions that might not be directly associated with cerebellar dysfunctions [91, 102]. Five consecutive days of anodal tDCS over the medial cerebellum, while not affecting patients’ mood evaluations, enhanced the recognition of specific negative emotional states in patients with Parkinson’s disease [102], in line with brain stimulation studies on healthy individuals [53, 54]. This effect may depend on cerebellar tDCS affecting a widespread network that enhances the processing of emotionally salient stimuli, as those with negative valence, Similarly, the positive effects on impulse control observed in borderline personality disorder patients following low-frequency rTMS over the left cerebellar hemisphere might be due to the stimulation exerting a facilitating effect on behavioral control mechanisms tapping on prefrontal regions through the modulation of cerebello-prefrontal connections [91].
Considerations and Recommendations
The reviewed studies present high heterogeneity in their methodology, in terms of different experimental designs, outcome measures, cerebellar target sites as well as stimulation parameters, including coil type (for TMS), and electrode montage and size (for tDCS). On one hand, this discrepancy may explain some inconsistency in the overall results, on the other hand, it complicates the identification of the stimulation protocols that may be more effective in boosting socio-affective functions. Nevertheless, despite this variability and the scarce literature on hereditary ataxia patients on this topic, the present review provides encouraging perspectives on the possibility of using cerebellar neurostimulation to improve the ability to process others’ mental states in healthy individuals and reduce social and affective symptoms in some neurological and psychiatric populations with cerebellar damage or with impairments in functions that involve the cerebellum. Yet, we cannot exclude that the presence of publication bias may have influenced our conclusions, leading to an overestimation of the benefits of NIBS due to a reduced tendency to disseminate null results. Therefore, the interpretations provided here must be taken with caution while awaiting both correctly powered and replication studies. Another important consideration is that, although defining a time window is mandatory when conducting a systematic review to allow the reproducibility of results, it also means missing to include relevant, recently published studies that could be beneficial for the review (see for instance [138,139,140]).
Furthermore, most of the reviewed studies targeted medial cerebellar regions, aiming at Crus I/II, in line with neuroimaging evidence reporting the functional connections between these sectors and the salience network [26, 27, 141], dedicated to the detection and attentional orientation towards emotional/salient stimuli [142], to select the more appropriate emotional response based on the individual’s current state (for a review see [143]). Only few articles targeted lateral sectors, in both the left [53, 83, 85, 87, 91] and right hemispheres [83, 84, 93, 100]. However, whereas low-level social operations, including the processing of others’ emotional expressions and one’s own affect regulation, may recruit medial regions [144], more complex social functions, including those involved in the processing of social sequences to predict higher-level mental states, such as beliefs, may be localized in slightly more lateral sectors [145], see also [139]). Accordingly, TMS “virtual lesion” studies showed that more lateral hemispheric regions are causally involved in processing others’ action intention [87], and others’ emotional and mental states inferences [53, 84, 85]. In light of the potential functional distinctionof social-affective operations along the medial–lateral axis of the cerebellum [144, 145], future studies are needed to test the effect of lateral cerebellar stimulation in boosting socio-affecting functions, which may be particularly effective in reducing patients’ difficulties with more complex and high-level social inferences. On this point, it is worth noting that among the reviewed articles, only 7 used individual MRI [83, 88, 92] or estimated-MRI [53, 84, 85, 87], to localize the target regions, in all other cases, the regions of interest were localized by using craniometrics points, which provide a less precise localization. Moreover, in the tDCS studies, the stimulation was applied through two relatively large electrodes (i.e., at least 25 cm2) that, although effective in modulating cerebellar activity, have low spatial resolution with stimulation spreading to at least part of the hemispheres [146, 147]. Hence, while the neurostimulation of the medial cerebellum seems promising in boosting socio-affective functions, it is not possible to draw sufficient conclusions regarding its specificity and the potential role of hemispheric regions. There is a need for research applying more precise localization methods such as individual MRI, computational modeling of the electric field distribution and, for tDCS studies, appropriate montage solutions using either smaller electrodes [148] or High-Definition tDCS (e.g., [149]) to improve stimulation focality. If this issue is relevant when targeting all cerebral regions, it is even more important for cerebellar stimulation, considering the convoluted structure of the cerebellar cortex, and particularly in conditions of increased atrophy or sulci width alterations, as in the case of hereditary ataxia. Moreover, future studies should evaluate the effects of the modulation of distinct cerebellar sectors through a comprehensive set of socio-affective outcome measures. Specifically to obtain a clearer view of the best cerebellar sector to target to potentiate distinct socio-affective functions, affect regulation abilities should be assessed using both questionnaire and experimental performance tasks, whereas tasks varying for complexity and abstraction of the required inferences should be employed to systematically evaluate the ability to process others’ mental states. Another possibility that future studies may address is the application of frequency-tuned stimulation for the treatment of diseases manifesting with abnormal cerebellar oscillatory activity. This approach, for which both TMS and specific types of transcranial electrical stimulation (such as transcranial alternating current stimulation, tACS) can be used, consists of “entraining” specific frequencies in the endogenous brain oscillatory activity that is associated with a specific function (see [150, 151]) and it is effective in modulating cerebellar excitability in a time- and frequency-dependent manner (e.g., [152,153,154]).
Lastly, all future research would certainly benefit from a more in-depth investigation of the precise neurophysiological mechanisms underlying the effects of cerebellar neurostimulation, which are currently not fully understood. Indeed, although cerebellar TMS induces both local as well as distal neurophysiological effects (see [155, 156]), driving synchronization of cerebello-cortical and cortico-cortical networks [157], it is still unclear which neural structures in the cerebellar cortex are most susceptible to stimulation [158]. It has been suggested that the modulation of cerebellar excitability involves long-term depression (LTD) and long-term potentiation (LTP) associated with local synaptic processes at the level of inhibitory Purkinje cells [158, 159]. However, another possibility is that Purkinje cells are stimulated trans-synaptically through parallel or climbing fibers [158], an option that deserves further consideration. Yet, evidence shows that TMS modulates cerebellar physiology also through facilitatory/inhibitory effects on excitatory granule cells and GABA-ergic interneurons [160]. Similarly, the electric field induced via cerebellar tDCS is suggested to polarize the superficial cortical layer that includes the large Purkinje cells [159, 161], but it is also likely to affect other neural elements in the cerebellar cortex, including granule and inhibitory cells, as well as climbing and mossy fibers, which explains why the direction of the physiological tDCS effect is difficult to predict [159]. Therefore, while cerebellar stimulation reliably induces behavioral and physiological modulation as evidenced by several controlled studies, including those presented here, further research is still required to appreciate the neurophysiological mechanisms at play, which would provide valuable insights to translate this knowledge into clinical applications for patients with hereditary ataxia.
Conclusions
In recent years, the cerebellum has increasingly attracted scientists interested in basic and clinical research of neuromodulation. Cerebellar alterations are related to significant difficulties in the ability to regulate one’s affects and to infer and understand others’ mental states both in hereditary ataxia patients and in other clinical conditions. Nevertheless, research on potential treatments to improve socio-affective abilities in these populations is currently lacking. NIBS, including TMS and tDCS, have been deemed as an effective treatment strategy for several mental conditions (for a meta-analysis see [162]). Indeed, consistent evidence points to the efficacy of NIBS in treating core symptoms and cognitive functions in different neurological and neuropsychiatric disorders as well as in improving behavioral and socio-affective deficits (see [163, 164]). Thus, the manipulation of cerebro-cerebellar circuits through NIBS, by modulating behavior as well as cognitive and socio-affective functions, offers an opportunity to explore therapeutic interventions that could ameliorate cognitive and affective deficits in hereditary ataxia patients.
Despite the scant evidence applying cerebellar NIBS to boost socio-affective functions in this specific population, the studies reviewed here support the potential efficacy of different cerebellar neurostimulation protocols in modulating mentalizing functions in healthy individuals and reducing affective and social symptoms in neurologic and psychiatric conditions associated with cerebellar alterations. Based on the hypothesis that conceives the cerebellum as a predictive device in social and affective functions, such a beneficial effect may depend on the stimulation boosting the formation of internal models of physical and social events and the implicit learning of regularities in other individuals’ behavior. Future research should clarify the cerebello-cerebral networks causally involved in affective and social behavior as well as the specific functional operations implemented by these circuits.
Data Availability
This declaration is not applicable.
Abbreviations
- ABC:
-
Aberrant Behavior Checklist
- ASD:
-
Autism spectrum disorder
- CCAS:
-
Cerebellar Cognitive Affective Syndrome
- CCASS:
-
Cerebellar Cognitive Affective Syndrome Scale
- CDSS:
-
Calgary Depression Scale for Schizophrenia
- CGI:
-
Clinical Global Impressions scale
- cTBS:
-
Continuous theta-burst stimulation
- dlPFC:
-
Dorsolateral prefrontal cortex
- dmPFC:
-
Dorsomedial prefrontal cortex
- GM:
-
Gray matter
- IAPS:
-
International Affective Picture System
- iTBS:
-
Intermittent theta-burst stimulation
- MADRS:
-
Montgomery Asberg Depression Rating Scale
- MRI:
-
Magnetic resonance images
- MT:
-
Motor threshold
- NIBS:
-
Non-Invasive Brain Stimulation
- PANAS:
-
Positive and Negative Affect Schedule
- PANSS:
-
Positive and Negative Syndrome Scale
- PHQ-9:
-
Patient Health Questionnaire-9
- POMS:
-
Profile of Mood States
- RMET:
-
Reading the Mind in the Eyes test
- rTMS:
-
Repetitive Transcranial Magnetic Stimulation
- SCA:
-
Spinocerebellar ataxia
- SF-36:
-
Short Form Health Survey 36
- SRT:
-
Serial Reaction Time
- STS:
-
Superior Temporal Sulcus
- tACS:
-
Transcranial alternating current stimulation
- TBS:
-
Theta-burst stimulation
- tDCS:
-
Transcranial Direct Current Stimulation
- TMS:
-
Transcranial Magnetic Stimulation
- ToM:
-
Theory of Mind
- TPJ:
-
Temporoparietal junction
- VAS:
-
Visual Analogue Scale
References
Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC. Verhoeven. J Consensus paper: cerebellum and emotion Cerebellum. 2017;16(2):552–76. https://doi.org/10.1007/s12311-016-0815-8.
Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JD, ..., Leggio M. Consensus paper: cerebellum and social cognition. Cerebellum. 2020;19(6):833–868. https://doi.org/10.1007/s12311-020-01155-1.
Lupo M, Olivito G, Gragnani A, Saettoni M, Siciliano L, Pancheri C, ..., Leggio M. Comparison of cerebellar grey matter alterations in bipolar and cerebellar patients: evidence from voxel-based analysis. Int J Mol Sci. 2021;22(7):3511. https://doi.org/10.3390/ijms22073511.
Lupo M, Olivito G, Siciliano L, Masciullo M, Bozzali M, Molinari M, Leggio M. Development of a psychiatric disorder linked to cerebellar lesions. Cerebellum. 2018;17:438–46. https://doi.org/10.1007/s12311-018-0926-5.
Mier D, Lis S, Neuthe K, Sauer C, Esslinger C, Gallhofer B, Kirsch P. The involvement of emotion recognition in affective theory of mind. Psychophysiology. 2010;47(6):1028–39. https://doi.org/10.1111/j.1469-8986.2010.01031.x.
Beer JS, Mitchell JP, Ochsner KN. Multiple perspectives on the psychological and neural bases of social cognition. Brain Res. 2006;1079:1–3. https://doi.org/10.1016/j.brainres.2006.02.001.
Fiske ST, Taylor SE. Social cognition. Mcgraw-Hill Book Company. 1991.
Brothers L, Ring B. A neuroethological framework for the representation of minds. J Cogn Neurosci. 1992;4(2):107–18. https://doi.org/10.1162/jocn.1992.4.2.107.
Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1(4):515–26.
Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol. 2018;154:59–70. https://doi.org/10.1016/B978-0-444-63956-1.00004-7.
D’Angelo E, Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Front Neural Circuits. 2013;6:116. https://doi.org/10.3389/fncir.2012.00116.
Leggio M, Molinari M. Cerebellar sequencing: a trick for predicting the future. Cerebellum. 2015;14:35–8. https://doi.org/10.1007/s12311-014-0616-x.
Clausi S, Olivito G, Lupo M, Siciliano L, Bozzali M, Leggio M. The cerebellar predictions for social interactions: theory of mind abilities in patients with degenerative cerebellar atrophy. Front Cell Neurosci. 2019;12:510. https://doi.org/10.3389/fncel.2018.00510.
Van Overwalle F, Manto M, Leggio M, Delgado-García JM. The sequencing process generated by the cerebellum crucially contributes to social interactio62ns. Med Hypotheses. 2019;128:33–42. https://doi.org/10.1016/j.mehy.2019.05.014.
Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290–4. https://doi.org/10.1080/14734220500348584.
Schmahmann JD, Weilburg JB, Sherman JC. The Neuropsychiatry of the Cerebellum - Insights from the Clinic. Cerebellum. 2007;6:254–67. https://doi.org/10.1080/14734220701490995.
Olivito G, Siciliano L, Clausi S, Lupo M, Baiocco R, Gragnani A, Saettoni M, Delle Chiaie R, Laghi F, Leggio M. The Cerebellum Gets Social: Evidence from an Exploratory Study of Cerebellar, Neurodevelopmental, and Psychiatric Disorders. Biomedicines. 2023;11(2):309. https://doi.org/10.3390/biomedicines11020309.
Arioli M, Crespi C, Canessa N. Social cognition through the lens of cognitive and clinical neuroscience. Biomed Res Int. 2018;2018:1–18. https://doi.org/10.1155/2018/4283427.
Heleven E, Van Overwalle F. The neural basis of representing others’ inner states. Curr Opin Psychol. 2018;23:98–103. https://doi.org/10.1016/j.copsyc.2018.02.003.
Van Overwalle F, Baetens K, Mariën P, Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–72. https://doi.org/10.1016/j.neuroimage.2013.09.033.
Van Overwalle F, Mariën P. Functional connectivity between the cerebrum and cerebellum in social cognition: a multi-study analysis. Neuroimage. 2016;124:248–55. https://doi.org/10.1016/j.neuroimage.2015.09.001.
Aichhorn M, Perner J, Weiss B, Kronbichler M, Staffen W, Ladurner G. Temporo-parietal junction activity in theory-of-mind tasks: falseness, beliefs, or attention. J Cogn Neurosci. 2009;21(6):1179–92. https://doi.org/10.1162/jocn.2009.21082.
Saxe R, Kanwisher N. People thinking about thinking people: the role of the temporo-parietal junction in “theory of mind”. In Social neuroscience, Psychology Press. 2013:pp. 171–182. https://doi.org/10.1016/s1053-8119(03)00230-1.
Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–98. https://doi.org/10.1002/(SICI)1097-0193(1996)4:3%3c174::AID-HBM3%3e3.0.CO;2-0.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34. https://doi.org/10.1146/annurev.neuro.31.060407.125606.
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45. https://doi.org/10.1152/jn.00339.2011.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29(26):8586–94. https://doi.org/10.1523/JNEUROSCI.1868-09.2009.
Sacchetti B, Scelfo B, Strata P. The cerebellum: synaptic changes and fear conditioning. Neuroscientist. 2005;11(3):217–27. https://doi.org/10.1177/1073858405276428.
Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20(3):445–68. https://doi.org/10.1016/s0896-6273(00)80987-3.
Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–87. https://doi.org/10.1016/j.neuron.2005.09.025.
Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11(2):157–66. https://doi.org/10.1006/nimg.1999.0525.
Calarge C, Andreasen NC, O’Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160(11):1954–64. https://doi.org/10.1176/appi.ajp.160.11.1954.
Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1(2):136–52. https://doi.org/10.1162/jocn.1989.1.2.136.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain: J Neurol. 1998;121(4):561–579. https://doi.org/10.1093/brain/121.4.561.
Tedesco AM, Chiricozzi FR, Clausi S, Lupo M, Molinari M, Leggio MG. The cerebellar cognitive profile. Brain. 2011;134(12):3672–86. https://doi.org/10.1093/brain/awr266.
Sokolov AA. The cerebellum in social cognition. Front Cell Neurosci. 2018;12:145. https://doi.org/10.3389/fncel.2018.00145.
Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304–13. https://doi.org/10.1038/nrn2332.
Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. https://doi.org/10.1016/s0074-7742(08)60346-3.
Clausi S, Olivito G, Siciliano L, Lupo M, Bozzali M, Masciullo M, ..., Leggio M. The neurobiological underpinning of the social cognition impairments in patients with spinocerebellar ataxia type 2. Cortex. 2021:138:101–112. https://doi.org/10.1016/j.cortex.2020.12.027.
Olivito G, Siciliano L, Clausi S, Lupo M, Romano S, Masciullo M, ..., Leggio M. Functional changes of mentalizing network in SCA2 patients: novel insights into understanding the social cerebellum. Cerebellum. 2020;19:235–242. https://doi.org/10.1007/s12311-019-01081-x.
Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs). Cerebellum. 2005;4:2–6. https://doi.org/10.1080/14734220510007914.
Bürk K, Globas C, Bösch S, Klockgether T, Zühlke C, Daum I, Dichgans J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J Neurol. 2003;250:207–11. https://doi.org/10.1007/s00415-003-0976-5.
Le Pira F, Zappalà G, Saponara R, Domina E, Restivo DA, Reggio E, ..., Giuffrida S. Cognitive findings in spinocerebellar ataxia type 2: relationship to genetic and clinical variables. J Neurol Sci. 2002;201(1–2):53–57. https://doi.org/10.1016/s0022-510x(02)00194-6.
Stone J, Smith L, Watt K, Barron L, Zeman A. Incoordinated thought and emotion in spinocerebellar ataxia type 8. J Neurol. 2001;248(3):229. https://doi.org/10.1007/s004150170232.
D’Agata F, Caroppo P, Baudino B, Caglio M, Croce M, Bergui M, ..., Orsi L. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum. 2011:10:600–610. https://doi.org/10.1007/s12311-011-0276-z.
Hoche F, Guell X, Sherman JC, Vangel MG, Schmahmann JD. Cerebellar contribution to social cognition. Cerebellum. 2016;15:732–43. https://doi.org/10.1007/s12311-015-0746-9.
Sokolovsky N, Cook A, Hunt H, Giunti P, Cipolotti L. A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 2, 1, and 7. Behav Neurol. 2010;23(1–2):17–29. https://doi.org/10.3233/BEN-2010-0270.
Blair RJ, Cipolotti L. Impaired social response reversal: A case of acquired sociopathy’. Brain. 2000;123(6):1122–41. https://doi.org/10.1093/brain/123.6.1122.
Garrard P, Martin NH, Giunti P, Cipolotti L. Cognitive and social cognitive functioning in spinocerebellar ataxia: a preliminary characterization. J Neurol. 2008;255:398–405. https://doi.org/10.1007/s00415-008-0680-6.
Ekman P, Friesen WV. Constants across cultures in the face and emotion. J Pers Soc Psychol. 1971;17(2):124. https://doi.org/10.1037/h0030377.
Tamietto M, Adenzato M, Geminiani G, de Gelder B. Fast recognition of social emotions takes the whole brain: interhemispheric cooperation in the absence of cerebral asymmetry. Neuropsychologia. 2007;45(4):836–43. https://doi.org/10.1016/j.neuropsychologia.2006.08.012.
Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The, “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001;42(2):241–51.
Ferrari C, Ciricugno A, Battelli L, Grossman ED, Cattaneo Z. Distinct cerebellar regions for body motion discrimination. Soc Cogn Affect Neurosci. 2022;17(1):72–80. https://doi.org/10.1093/scan/nsz088.
Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, ..., Priori A. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cogn Emot. 2012;26(5):786–799. https://doi.org/10.1080/02699931.2011.619520.
Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10(5):640–56. https://doi.org/10.1162/089892998562942.
Tamaš O, Kostić M, Kačar A, Stefanova E, Ðokić BS, Stanisavljević D, ..., Dragašević-Mišković N. Social cognition in patients with cerebellar neurodegenerative disorders. Front Syst Neurosci. 2021;15:664223. https://doi.org/10.3389/fnsys.2021.664223.
Giocondo F, Curcio G. Spinocerebellar ataxia: a critical review of cognitive and socio-cognitive deficits. Int J Neurosci. 2018;128(2):182–91. https://doi.org/10.1080/00207454.2017.1377198.
Antal A, Luber B, Brem AK, Bikson M, Brunoni AR, Kadosh RC, ..., Paulus W. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract. 2022;7:146–165. https://doi.org/10.1016/j.cnp.2022.05.002.
Rothwell J. Transcranial brain stimulation: past and future. Brain Neurosci Adv. 2018;2:1–4. https://doi.org/10.1177/2398212818818070.
Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: a neurochronometrics of mind, MIT press, 2003.
Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage. 2014;85:961–70. https://doi.org/10.1016/j.neuroimage.2013.06.007.
Romei V, Thut G, Silvanto J. Information-based approaches of noninvasive transcranial brain stimulation. Trends Neurosci. 2016;39(11):782–95. https://doi.org/10.1016/j.tins.2016.09.001.
Silvanto J, Cattaneo Z. Common framework for “virtual lesion” and state-dependent TMS: the facilitatory/suppressive range model of online TMS effects on behavior. Brain Cogn. 2017;119:32–8. https://doi.org/10.1016/j.bandc.2017.09.007.
Silvanto J, Cattaneo Z. Nonlinear interaction between stimulation intensity and initial brain state: Evidence for the facilitatory/suppressive range model of online TMS effects. Neurosci Lett. 2021;742:135538. https://doi.org/10.1016/j.neulet.2020.135538.
Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol. 2006;117(12):2584–96. https://doi.org/10.1016/j.clinph.2006.06.712.
Deng ZD, Lisanby SH, Peterchev AV. Electric field depth–focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 2013;6(1):1–13. https://doi.org/10.1016/j.brs.2012.02.005.
Cattaneo Z, Bona S, Ciricugno A, Silvanto J. The chronometry of symmetry detection in the lateral occipital (LO) cortex. Neuropsychologia. 2022;167:108160. https://doi.org/10.1016/j.neuropsychologia.2022.108160.
de Graaf TA, Koivisto M, Jacobs C, Sack AT. The chronometry of visual perception: review of occipital TMS masking studies. Neurosci Biobehav Rev. 2014;45:295–304. https://doi.org/10.1016/j.neubiorev.2014.06.017.
Beynel L, Appelbaum LG, Luber B, Crowell CA, Hilbig SA, Lim W, ..., Deng ZD. Effects of online repetitive transcranial magnetic stimulation (rTMS) on cognitive processing: a meta-analysis and recommendations for future studies. Neurosci Biobehav Rev. 2019;107:47–58. https://doi.org/10.1016/j.neubiorev.2019.08.018.
Stagg CJ, Antal A, Nitsche MA. Physiology of transcranial direct current stimulation. J Ect. 2018;34(3):144–52. https://doi.org/10.1097/YCT.0000000000000510.
Benwell CS, Learmonth G, Miniussi C, Harvey M, Thut G. Non-linear effects of transcranial direct current stimulation as a function of individual baseline performance: Evidence from biparietal tDCS influence on lateralized attention bias. Cortex. 2015;69:152–65. https://doi.org/10.1016/j.cortex.2015.05.007.
Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216(1):1–10. https://doi.org/10.1007/s00221-011-2891-9.
Oldrati V, Schutter DJ. Targeting the human cerebellum with transcranial direct current stimulation to modulate behavior: a meta-analysis. Cerebellum. 2018;17(2):228–36. https://doi.org/10.1007/s12311-017-0877-2.
Thair H, Holloway AL, Newport R, Smith AD. Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci. 2017;11:641. https://doi.org/10.3389/fnins.2017.00641.
Jamil A, Batsikadze G, Kuo HI, Labruna L, Hasan A, Paulus W, Nitsche MA. Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. J Physiol. 2017;595(4):1273–88. https://doi.org/10.1113/JP272738.
Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? J Psychiatry Neurosci. 2005;30(3):178–86. https://doi.org/10.3389/fpubh.2015.00066.
López-Bastida J, Perestelo-Pérez L, Montón-Alvarez F, Serrano-Aguilar P. Social economic costs and health-related quality of life in patients with degenerative cerebellar ataxia in Spain. Mov Disord. 2008;23(2):212–7. https://doi.org/10.1002/mds.21798.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26. https://doi.org/10.11124/JBIES-20-00167.
Buscemi N, Hartling L, Vandermeer B, Tjosvold L, Klassen TP. Single data extraction generated more errors than double data extraction in systematic reviews. J Clin Epidemiol. 2006;59(7):697–703. https://doi.org/10.1016/j.jclinepi.2005.11.010.
Schutter DJ, van Honk J, d’Alfonso AA, Peper JS, Panksepp J. High frequency repetitive transcranial magnetic over the medial cerebellum induces a shift in the prefrontal electroencephalography gamma spectrum: a pilot study in humans. Neurosci Lett. 2003;336(2):73–6. https://doi.org/10.1016/s0304-3940(02)01077-7.
Schutter DJ, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8:28–34. https://doi.org/10.1007/s12311-008-0056-6.
Schutter DJ, Enter D, Hoppenbrouwers SS. High-frequency repetitive transcranial magnetic stimulation to the cerebellum and implicit processing of happy facial expressions. J Psychiatry Neurosci. 2009;34(1):60–5.
Demirtas-Tatlidede A, Freitas C, Pascual-Leone A, Schmahmann JD. Modulatory effects of theta burst stimulation on cerebellar nonsomatic functions. Cerebellum. 2011;10:495–503. https://doi.org/10.1007/s12311-010-0230-5.
Gamond L, Ferrari C, La Rocca S, Cattaneo Z. Dorsomedial prefrontal cortex and cerebellar contribution to in-group attitudes: a transcranial magnetic stimulation study. Eur J Neurosci. 2017;45(7):932–9. https://doi.org/10.1111/ejn.13529.
Ferrari C, Oldrati V, Gallucci M, Vecchi T, Cattaneo Z. The role of the cerebellum in explicit and incidental processing of facial emotional expressions: a study with transcranial magnetic stimulation. Neuroimage. 2018;169:256–64. https://doi.org/10.1016/j.neuroimage.2017.12.026.
Heleven E, Van Dun K, De Witte S, Baeken C, Van Overwalle F. The role of the cerebellum in social and non-social action sequences: a preliminary LF-rTMS study. Front Hum Neurosci. 2021;15:593821. https://doi.org/10.3389/fnhum.2021.593821.
Ferrari C, Ciricugno A, Urgesi C, Cattaneo Z. Cerebellar contribution to emotional body language perception: a TMS study. Soc Cogn Affect Neurosci. 2022;17(1):81–90. https://doi.org/10.1093/scan/nsz074.
Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, ..., Pascual-Leone A. Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res. 2010;124(1–3):91–100. https://doi.org/10.1016/j.schres.2010.08.015.
Tikka SK, Garg S, Sinha VK, Nizamie SH, Goyal N. Resting state dense array gamma oscillatory activity as a response marker for cerebellar-repetitive transcranial magnetic stimulation (rTMS) in schizophrenia. J Ect. 2015;31(4):258–62. https://doi.org/10.1097/YCT.0000000000000242.
Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N. The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiat Res. 2016;243:413–20. https://doi.org/10.1016/j.psychres.2016.07.023.
De Vidovich GZ, Muffatti R, Monaco J, Caramia N, Broglia D, Caverzasi E, ..., D’Angelo E. Repetitive TMS on left cerebellum affects impulsivity in borderline personality disorder: A pilot study. Front Hum Neurosci. 2016;10:582. https://doi.org/10.3389/fnhum.2016.00582.
Brady Jr RO, Gonsalvez I, Lee I, Öngür D, Seidman LJ, Schmahmann JD, ..., Halko MA. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiat. 2019;176(7):512–520. https://doi.org/10.1176/appi.ajp.2018.18040429.
Newstead S, Young H, Benton D, Jiga-Boy G, Andrade Sienz ML, Clement RM, Boy F. Acute and repetitive fronto-cerebellar tDCS stimulation improves mood in non-depressed participants. Exp Brain Res. 2018;236:83–97. https://doi.org/10.1007/s00221-017-5109-y.
Gheorghe DA, Panouillères MT, Walsh ND. Investigating the effects of cerebellar transcranial direct current stimulation on saccadic adaptation and cortisol response. Cerebellum Ataxias. 2021;8:1–11. https://doi.org/10.1186/s40673-020-00124-y.
Oldrati V, Ferrari E, Butti N, Cattaneo Z, Borgatti R, Urgesi C, Finisguerra A. How social is the cerebellum? Exploring the effects of cerebellar transcranial direct current stimulation on the prediction of social and physical events. Brain Struct Funct. 2021;226(3):671–84. https://doi.org/10.1007/s00429-020-02198-0.
Clausi S, Lupo M, Funghi G, Mammone A, Leggio M. Modulating mental state recognition by anodal tDCS over the cerebellum. Sci Rep. 2022;12(1):22616. https://doi.org/10.1038/s41598-022-26914-4.
Ma Q, Pu M, Li M, Haihambo N, Baetens K, Heleven E, ..., Van Overwalle F. Can transcranial direct current stimulation (tDCS) of the cerebellum improve implicit social and cognitive sequence learning?. Int J Clin Health Psychol. 2023;23(2):100355. https://doi.org/10.1016/j.ijchp.2022.100355.
Ho KA, Bai S, Martin D, Alonzo A, Dokos S, Puras P, Loo CK. A pilot study of alternative transcranial direct current stimulation electrode montages for the treatment of major depression. J Affect Disord. 2014;167:251–8. https://doi.org/10.1016/j.jad.2014.06.022.
Benussi A, Cantoni V, Manes M, Libri I, Dell’Era V, Datta A, ..., Borroni B. Motor and cognitive outcomes of cerebello-spinal stimulation in neurodegenerative ataxia. Brain. 2021;144(8):2310–2321. https://doi.org/10.1093/brain/awab157.
D’Urso G, Toscano E, Sanges V, Sauvaget A, Sheffer CE, Riccio MP, ..., de Bartolomeis A. Cerebellar transcranial direct current stimulation in children with autism spectrum disorder: a pilot study on efficacy, feasibility, safety, and unexpected outcomes in Tic disorder and epilepsy. J Clin Med. 2021;11(1):143. https://doi.org/10.3390/jcm11010143.
Maas RP, Teerenstra S, Toni I, Klockgether T, Schutter DJ, van de Warrenburg BP. Cerebellar transcranial direct current stimulation in spinocerebellar ataxia type 3: a randomized, double-blind, sham-controlled trial. Neurotherapeutics. 2022;19(4):1259–72. https://doi.org/10.1007/s13311-022-01231-w.
Ruggiero F, Dini M, Cortese F, Vergari M, Nigro M, Poletti B, ..., Ferrucci R. Anodal transcranial direct current stimulation over the cerebellum enhances sadness recognition in Parkinson’s disease patients: a pilot study. Cerebellum. 2022;21(2):234–243. https://doi.org/10.1007/s12311-021-01295-y.
Renzi C, Vecchi T, D’Angelo E, Silvanto J, Cattaneo Z. Phosphene induction by cerebellar transcranial magnetic stimulation. Clin Neurophysiol. 2014;125(10):2132–3. https://doi.org/10.1016/j.clinph.2014.01.031.
Hardwick RM, Lesage E, Miall RC. Cerebellar transcranial magnetic stimulation: the role of coil geometry and tissue depth. Brain Stimul. 2014;7(5):643–9. https://doi.org/10.1016/j.brs.2014.04.009.
Sack AT, Kadosh RC, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21(2):207–21. https://doi.org/10.1162/jocn.2009.21126.
Moberget T, Ivry RB. Prediction, psychosis, and the cerebellum. Biol Psychiat Cogn Neurosci Neuroimaging. 2019;4(9):820–31. https://doi.org/10.1016/j.bpsc.2019.06.001.
Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health. 2015;66:1–8. https://doi.org/10.3389/fpubh.2015.00066.
Matosin N, Frank EM, Engel M, Lum JS, Newell KA. Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis Model Mech. 2014;7(2):171–173. https://doi.org/10.1242/dmm.015123.
Buchanan DM, Bogdanowicz T, Khanna N, Lockman-Dufour G, Robaey P, D’Angiulli A. Systematic Review on the Safety and Tolerability of Transcranial Direct Current Stimulation in Children and Adolescents. Brain Sci. 2021;11(2):212. https://doi.org/10.3390/brainsci11020212.
Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, … Paulus W. Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clin Neurophysiol. 2017;128(9):1774–1809. https://doi.org/10.1016/j.clinph.2017.06.001.
Day SJ, Altman DG. Blinding in clinical trials and other studies. BMJ. 2000;321(7259):504. https://doi.org/10.1136/bmj.321.7259.504.
Wang X, Ji X. Sample size estimation in clinical research: from randomized controlled trials to observational studies. Chest. 2020;158(1):S12–20. https://doi.org/10.1016/j.chest.2020.03.010.
Sullivan GM, Feinn R. Using effect size—or why the P value is not enough. J Grad Med Educ. 2012;4(3):279–82. https://doi.org/10.4300/JGME-D-12-00156.1.
Depping MS, Schmitgen MM, Kubera KM, Wolf RC. Cerebellar contributions to major depression. Front Psychiatry. 2018;9:634. https://doi.org/10.3389/fpsyt.2018.00634.
Mapelli L, Soda T, D’Angelo E, Prestori F. The cerebellar involvement in autism spectrum disorders: from the social brain to mouse models. Int J Mol Sci. 2022;23(7):3894. https://doi.org/10.3390/ijms23073894.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136(3):696–709. https://doi.org/10.1093/brain/aws360.
Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving activities of daily living, and physical and cognitive functioning, in people after stroke. Cochrane Database Syst Rev. 2020;11(11). https://doi.org/10.1002/14651858.CD009645.pub4.
Wang J, Luo H, Schülke R, Geng X, Sahakian BJ, Wang S. Is transcranial direct current stimulation, alone or in combination with antidepressant medications or psychotherapies, effective in treating major depressive disorder? A systematic review and meta-analysis. BMC Med. 2021;19(1):1–14. https://doi.org/10.1186/s12916-021-02181-4.
Baumann O, Mattingley JB. Functional topography of primary emotion processing in the human cerebellum. Neuroimage. 2012;61(4):805–11. https://doi.org/10.1016/j.neuroimage.2012.03.044.
Schraa-Tam CK, Rietdijk WJ, Verbeke WJ, Dietvorst RC, van den Berg WE, Bagozzi RP, De Zeeuw CI. fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum. 2012;11:233–45. https://doi.org/10.1007/s12311-011-0301-2.
De Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci. 2004;101(47):16701–6. https://doi.org/10.1073/pnas.0407042101.
Siman-Tov T, Granot RY, Shany O, Singer N, Hendler T, Gordon CR. Is there a prediction network? Meta-analytic evidence for a cortical-subcortical network likely subserving prediction. Neurosci Biobehav Rev. 2019;105:262–75. https://doi.org/10.1016/j.neubiorev.2019.08.012.
Ma Q, Pu M, Heleven E, Haihambo NP, Baetens K, Baeken C, ..., Van Overwalle F. The posterior cerebellum supports implicit learning of social belief sequences. Cogn Affect Behav Neurosci. 2021;21(5):970–992. https://doi.org/10.3758/s13415-021-00910-z.
Van Overwalle F, Baeken C, Campanella S, Crunelle CL, Heleven E, Kornreich C, ..., Baetens K. The role of the posterior cerebellum in dysfunctional social sequencing. Cerebellum. 2021:1–12. https://doi.org/10.1007/s12311-021-01330-y.
Murphy DN, Boggio P, Fregni F. Transcranial direct current stimulation as a therapeutic tool for the treatment of major depression: insights from past and recent clinical studies. Curr Opin Psychiatry. 2009;22(3):306–11. https://doi.org/10.1097/YCO.0b013e32832a133f.
Schutter DJ. A cerebellar framework for predictive coding and homeostatic regulation in depressive disorder. Cerebellum. 2016;15(1):30–3. https://doi.org/10.1007/s12311-015-0708-2.
Balsters JH, Apps MA, Bolis D, Lehner R, Gallagher L, Wenderoth N. Disrupted prediction errors index social deficits in autism spectrum disorder. Brain. 2017;140(1):235–46. https://doi.org/10.1093/brain/aww287.
Sinha P, Kjelgaard MM, Gandhi TK, Tsourides K, Cardinaux AL, Pantazis D, ..., Held RM. Autism as a disorder of prediction. Proc Natl Acad Sci. 2014;111(42):15220–15225. https://doi.org/10.1073/pnas.1416797111.
DuBois D, Ameis SH, Lai MC, Casanova MF, Desarkar P. Interoception in autism spectrum disorder: A review. Int J Dev Neurosci. 2016;52:104–11. https://doi.org/10.1016/j.ijdevneu.2016.05.001.
Kelly E, Escamilla CO, Tsai PT. Cerebellar dysfunction in autism spectrum disorders: deriving mechanistic insights from an internal model framework. Neuroscience. 2021;462:274–87. https://doi.org/10.1016/j.neuroscience.2020.11.012.
Stoodley CJ, Tsai PT. Adaptive prediction for social contexts: the cerebellar contribution to typical and atypical social behaviors. Annu Rev Neurosci. 2021;44:475–93. https://doi.org/10.1146/annurev-neuro-100120-092143.
Liddle PF, Liddle EB. Imprecise Predictive Coding Is at the Core of Classical Schizophrenia. Front Hum Neurosci. 2022;104:1–16. https://doi.org/10.3389/fnhum.2022.818711The.
Ding Y, Ou Y, Pan P, Shan X, Chen J, Liu F, ..., Guo W. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging. 2019:283:24–33. https://doi.org/10.1016/j.pscychresns.2018.11.009.
He H, Luo C, Luo Y, Duan M, Yi Q, Biswal BB, Yao D. Reduction in gray matter of cerebellum in schizophrenia and its influence on static and dynamic connectivity. Hum Brain Mapp. 2019;40(2):517–28. https://doi.org/10.1002/hbm.24391.
Kim DJ, Kent JS, Bolbecker AR, Sporns O, Cheng H, Newman SD, ..., Hetrick WP. Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull. 2014;40(6):1216–1226. https://doi.org/10.1093/schbul/sbu059.
Zhu DM, Yang Y, Zhang Y, Wang C, Wang Y, Zhang C, ..., Zhu J. Cerebellar-cerebral dynamic functional connectivity alterations in major depressive disorder. J Affect Disord. 2020;275:319–328. https://doi.org/10.1016/j.jad.2020.06.062.
Kube T, Schwarting R, Rozenkrantz L, Glombiewski JA, Rief W. Distorted cognitive processes in major depression: a predictive processing perspective. Biol Psychiatry. 2020;87(5):388–98. https://doi.org/10.1016/j.biopsych.2019.07.017.
Bongaerts F, Klaus J, Terburg D, Schutter D. Trait aggression-dependent effects of cerebellar tDCS in a social dominance task. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation. 2023;16(1):2.
Ferrari C, Ciricugno A, Arioli M, Cattaneo Z. Functional segregation of the human cerebellum in social cognitive tasks revealed by TMS. J Neurosci. 2023;43(20):3708–17.
Malatesta G, D’Anselmo A, Prete G, Lucafò C, Faieta L, Tommasi L. The predictive role of the posterior cerebellum in the processing of dynamic emotions. Cerebellum. 2023;1–9.
Habas C, Manto M. Probing the neuroanatomy of the cerebellum using tractography. Handb Clin Neurol. 2018;154:235–49. https://doi.org/10.1016/B978-0-444-63956-1.00014-X.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, ..., Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007.
Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 2013;23(3):361–72. https://doi.org/10.1016/j.conb.2012.12.012.
Metoki A, Wang Y, Olson IR. The social cerebellum: a large-scale investigation of functional and structural specificity and connectivity. Cereb Cortex. 2022;32(5):987–1003. https://doi.org/10.1093/cercor/bhab260.
Kruithof ES, Klaus J, Schutter DJ. The cerebellum in aggression: Extending the cortico-limbic dual-route model of motivation and emotion. Motiv Sci. 2022;8(2):150. https://doi.org/10.1037/mot0000251.
Gomez-Tames J, Asai A, Mikkonen M, Laakso I, Tanaka S, Uehara S, ..., Hirata A. Group-level and functional-region analysis of electric-field shape during cerebellar transcranial direct current stimulation with different electrode montages. J Neural Eng. 2019;16(3):036001. https://doi.org/10.1088/1741-2552/ab0ac5.
Parazzini M, Rossi E, Ferrucci R, Liorni I, Priori A, Ravazzani P. Modelling the electric field and the current density generated by cerebellar transcranial DC stimulation in humans. Clin Neurophysiol. 2014;125(3):577–84. https://doi.org/10.1016/j.clinph.2013.09.039.
Klaus J, Schutter DJ. Electrode montage-dependent intracranial variability in electric fields induced by cerebellar transcranial direct current stimulation. Sci Rep. 2021;11(1):22183. https://doi.org/10.1038/s41598-021-01755-9.
Maldonado T, Bernard JA. The polarity-specific nature of single-session high-definition transcranial direct current stimulation to the cerebellum and prefrontal cortex on motor and non-motor task performance. Cerebellum. 2021;20(4):569–83. https://doi.org/10.1007/s12311-021-01235-w.
Hallett M, Di Iorio R, Rossini PM, Park JE, Chen R, Celnik P, ..., Ugawa Y. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clin Neurophysiol. 2017;128(11):2125–2139. https://doi.org/10.1016/j.clinph.2017.08.007.
Wessel MJ, Draaisma LR, Hummel FC. Mini-review: transcranial alternating current stimulation and the cerebellum. Cerebellum. 2023;22(1):120–8. https://doi.org/10.1007/s12311-021-01362-4.
Dave S, VanHaerents S, Voss JL. Cerebellar theta and beta noninvasive stimulation rhythms differentially influence episodic memory versus semantic prediction. J Neurosci. 2020;40(38):7300–10. https://doi.org/10.1523/JNEUROSCI.0595-20.2020.
Giustiniani A, Tarantino V, Bracco M, Bonaventura RE, Oliveri M. Functional role of cerebellar gamma frequency in motor sequences learning: a tACS study. Cerebellum. 2021;20(6):913–21. https://doi.org/10.1007/s12311-021-01255-6.
Spampinato D, Avci E, Rothwell J, Rocchi L. Frequency-dependent modulation of cerebellar excitability during the application of non-invasive alternating current stimulation. Brain Stimul. 2021;14(2):277–83. https://doi.org/10.1016/j.brs.2021.01.007.
Cho SS, Yoon EJ, Bang SA, Park HS, Kim YK, Strafella AP, Kim SE. Metabolic changes of cerebrum by repetitive transcranial magnetic stimulation over lateral cerebellum: a study with FDG PET. The Cerebellum. 2012;11:739–48.
Du X, Rowland LM, Summerfelt A, Choa FS, Wittenberg GF, Wisner K, ..., Hong LE. Cerebellar-stimulation evoked prefrontal electrical synchrony is modulated by GABA. The Cerebellum. 2018;17:550–563.
Tremblay SA, Chapman CA, Courtemanche R. State-dependent entrainment of prefrontal cortex local field potential activity following patterned stimulation of the cerebellar vermis. Front Syst Neurosci. 2019;13:60.
Siebner HR, Funke K, Aberra AS, Antal A, Bestmann S, Chen R, ..., Ugawa Y. Transcranial magnetic stimulation of the brain: What is stimulated?–a consensus and critical position paper. Clin Neurophysiol. 2022;140:59–97.
Klaus J, Schutter DJ. Non-invasive brain stimulation of the cerebellum in emotion. In: The Emotional Cerebellum. Cham: Springer International Publishing; 2022. p. 109–21.
Koch G. Repetitive transcranial magnetic stimulation: a tool for human cerebellar plasticity. Funct Neurol. 2010;25(3):159.
Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, ..., Celnik P. Cerebellar transcranial direct current stimulation (ctDCS) a novel approach to understanding cerebellar function in health and disease. Neuroscientist. 2016;22(1):83–97.
Hyde J, Carr H, Kelley N, et al. Efficacy of neurostimulation across mental disorders: systematic review and meta-analysis of 208 randomized controlled trials. Mol Psychiatry. 2022;27:2709–19.
Boggio PS, Asthana MK, Costa TL, Valasek CA, Osório AA. Promoting social plasticity in developmental disorders with non-invasive brain stimulation techniques. Front Neurosci. 2015;9:294.
To WT, De Ridder D, Hart J Jr, Vanneste S. Changing Brain Networks Through Non-invasive Neuromodulation. Front Hum Neurosci. 2018;12:128.
Funding
This research was funded by the Italian Ministry of Health by Ricerca Corrente 2021/2022/2023 to Z.C (IRCCS Mondino Foundation), M.L. (Fondazione Santa Lucia IRCCS) and C.U. (Scientific Institute IRCCS Eugenio Medea), Ricerca Finalizzata 2016 (GR- 2016–02363640 to Z.C. and C.U.), Ricerca Finalizzata 2021 (RF-2021–12374279 to A.C., M.L. and C.U.) as well as by Italian Ministry of University and Research (PRIN 20203LT7H3 to Z.C. and M.L.). This work was supported also by “Finanziamento dell’Unione Europea – NextGenerationEU – missione 4, componente 2, investimento 1.1.” PRIN PNRR (Project Code: P2022TWSWS - CUP: B53D23030350001) to G. O.

Author information
Authors and Affiliations
Contributions
Conceptualization, A.C., V.O., Z.C., M.L., C.U., and G.O.; methodology, A.C. and V.O..; formal analysis, A.C. and V.O.; investigation, A.C. and V.O.; data curation, A.C. and V.O.; writing—original draft preparation, A.C., V.O. and G.O.; writing—review and editing, A.C., V.O., Z.C., C.U., M.L. and G.O..; funding acquisition, A.C., Z.C., M.L. and C.U. The authors A.C. and V.O. equally contributed to the manuscript and shared first co-authorship. All authors have read and agreed to the current version of the manuscript.”
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciricugno, A., Oldrati, V., Cattaneo, Z. et al. Cerebellar Neurostimulation for Boosting Social and Affective Functions: Implications for the Rehabilitation of Hereditary Ataxia Patients. Cerebellum (2024). https://doi.org/10.1007/s12311-023-01652-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-023-01652-z