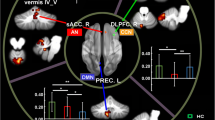
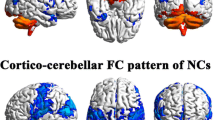
Abstract
Major depressive disorder (MDD) is a serious and widespread psychiatric disorder. Previous studies mainly focused on cerebrum functional connectivity, and the sample size was relatively small. However, functional connectivity is undirected. And, there is increasing evidence that the cerebellum is also involved in emotion and cognitive processing and makes outstanding contributions to the symptomology and pathology of depression. Therefore, we used a large sample size of resting-state functional magnetic resonance imaging (rs-fMRI) data to investigate the altered effective connectivity (EC) among the cerebellum and other cerebral cortex in patients with MDD. Here, from the perspective of data-driven analysis, we used two different atlases to divide the whole brain into different regions and analyzed the alterations of EC and EC networks in the MDD group compared with healthy controls group (HCs). The results showed that compared with HCs, there were significantly altered EC in the cerebellum-neocortex and cerebellum-basal ganglia circuits in MDD patients, which implied that the cerebellum may be a potential biomarker of depressive disorders. And, the alterations of EC brain networks in MDD patients may provide new insights into the pathophysiological mechanisms of depression.



Similar content being viewed by others
References
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50(5):668–81. https://doi.org/10.1038/s41588-018-0090-3.
Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–105. https://doi.org/10.1001/jama.289.23.3095.
Ho TC, Gutman B, Pozzi E, Grabe HJ, Hosten N, Wittfeld K, et al. Subcortical shape alterations in major depressive disorder: findings from the ENIGMA major depressive disorder working group. Hum Brain Mapp. 2022;43(1):341–51. https://doi.org/10.1002/hbm.24988.
Lemke H, Probst S, Warneke A, Waltemate L, Winter A, Thiel K, et al. The course of disease in major depressive disorder is associated with altered activity of the limbic system during negative emotion processing. Biol Psychiatry Cogn Neurosci Neuroimaging. 2022;7(3):323–32. https://doi.org/10.1016/j.bpsc.2021.05.008.
Zhang L, Verwer RWH, Zhao J, Huitinga I, Lucassen PJ, Swaab DF. Changes in glial gene expression in the prefrontal cortex in relation to major depressive disorder, suicide and psychotic features. J Affect Disord. 2021;295:893–903. https://doi.org/10.1016/j.jad.2021.08.098.
Lai CH. Fronto-limbic neuroimaging biomarkers for diagnosis and prediction of treatment responses in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2021;107:110234. https://doi.org/10.1016/j.pnpbp.2020.110234.
Adamaszek M, D’Agata F, Ferrucci R, Habas C, Keulen S, Kirkby KC, et al. Consensus paper: cerebellum and emotion. Cerebellum. 2017;16(2):552–76. https://doi.org/10.1007/s12311-016-0815-8.
Ruggiero F, Dini M, Cortese F, Vergari M, Nigro M, Poletti B, et al. Anodal transcranial direct current stimulation over the cerebellum enhances sadness recognition in Parkinson’s disease patients: a pilot study. The Cerebellum. 2022;21(2):234–43. https://doi.org/10.1007/s12311-021-01295-y.
Nguyen VT, Sonkusare S, Stadler J, Hu X, Breakspear M, Guo CC. Distinct cerebellar contributions to cognitive-perceptual dynamics during natural viewing. Cereb Cortex. 2017;27(12):5652–62. https://doi.org/10.1093/cercor/bhw334.
Wagner MJ, Luo L. Neocortex–cerebellum circuits for cognitive processing[J]. Trends Neurosci. 2020;43(1):42–54. https://doi.org/10.1016/j.tins.2019.11.002.
Li N, Mrsic-Flogel TD. Cortico-cerebellar interactions during goal-directed behavior[J]. Curr Opin Neurobiol. 2020;65:27–37. https://doi.org/10.1016/j.conb.2020.08.010.
Bostan AC, Strick PL. The basal ganglia and the cerebellum: nodes in an integrated network[J]. Nat Rev Neurosci. 2018;19(6):338–50. https://doi.org/10.1038/s41583-018-0002-7.
Habas C. Research Note: Functional connectivity between a corticostriatal network and the cerebellum. The Cerebellum. 2022;21(3):520–4. https://doi.org/10.1007/s12311-021-01316-w.
Pierce JE, Péron J. The basal ganglia and the cerebellum in human emotion. Soc Cogn Affect Neurosci. 2020;15(5):599–613. https://doi.org/10.1093/scan/nsaa076.
Hausman HK, Jackson TB, Goen JRM, Bernard JA. From synchrony to asynchrony: cerebellar–basal ganglia functional circuits in young and older adults. Cereb Cortex. 2020;30(2):718–29. https://doi.org/10.1093/cercor/bhz121.
Sacu S, Wackerhagen C, Erk S, Romanczuk-Seiferth N, Schwarz K, Schweiger J, et al. Effective connectivity during face processing in major depression–distinguishingmarkers of pathology, risk, and resilience. Psychological Medicine. 2022;1–13. https://doi.org/10.1017/S0033291722000824.
Yang L, Wei AH, Ouyang TT, Cao ZZ, Duan AW, Zhang HH. Functional plasticity abnormalities over the lifespan of first-episode patients with major depressive disorder: a resting state fMRI study. Annals of Translational Medicine. 2021;9(4):349. https://doi.org/10.21037/atm-21-367.
Xin Y, Bai T, Zhang T, Chen Y, Wang K, Yu S. Electroconvulsive therapy modulates critical brain dynamics in major depressive disorder patients. Brain Stimul. 2022;15(1):214–25. https://doi.org/10.1016/j.brs.2021.12.008.
Jung JY, Choi S, Han KM, Kim A, Kang W, Paik JW, et al. Alterations in functional brain networks in depressed patients with a suicide attempt history. Neuropsychopharmacology. 2020;45(6):964–74. https://doi.org/10.1038/s41386-019-0560-z.
Ishida T, Dierks T, Strik W, Morishima Y. Converging resting state networks unravels potential remote effects of transcranial magnetic stimulation for major depression. Front Psych. 2020;11:836. https://doi.org/10.3389/fpsyt.2020.00836.
Ji J, Zou A, Liu J, Yang C, Zhang X, Song Y. A Survey on brain effective connectivity network learning. IEEE Trans Neural Netw Learn Syst. 2021. https://doi.org/10.1109/TNNLS.2021.3106299.
Yan CG, Chen X, Li L, Castellanos FX, Bai TJ, Bo QJ, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci. 2019;116(18):9078–83. https://doi.org/10.1073/pnas.1900390116.
Rolls ET, Huang CC, Lin CP, Feng J, Joliot M. Automated anatomical labelling atlas 3. Neuroimage. 2020;206:116189. https://doi.org/10.1016/j.neuroimage.2019.116189.
Sun K, Liu Z, Chen G, Zhou Z, Zhong S, Tang Z. A two-center radiomic analysis for differentiating major depressive disorder using multi-modality MRI data under different parcellation methods. J Affect Disord. 2022;300:1–9. https://doi.org/10.1016/j.jad.2021.12.065.
Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods. 2012;203(2):418–26. https://doi.org/10.1016/j.jneumeth.2011.10.006.
Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–27. https://doi.org/10.1093/biostatistics/kxj037.
Fortin JP, Cullen N, Sheline YI, Taylor WD, Aselcioglu I, Cook PA, et al. Harmonization of cortical thickness measurements across scanners and sites. Neuroimage. 2018;167:104–20. https://doi.org/10.1016/j.neuroimage.2017.11.024.
Yu M, Linn KA, Cook PA, Phillips ML, McInnis M, Fava M, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum Brain Mapp. 2018;39(11):4213–27. https://doi.org/10.1002/hbm.24241.
Fortin JP, Parker D, Tunç B, Watanabe T, Elliott MA, Ruparel K, et al. Harmonization of multi-site diffusion tensor imaging data. Neuroimage. 2017;161:149–70. https://doi.org/10.1016/j.neuroimage.2017.08.047.
Bell TK, Godfrey KJ, Ware AL, Yeates KO, Harris AD. Harmonization of multi-site MRS data with ComBat. NeuroImage. 2022;257:119330. https://doi.org/10.1016/j.neuroimage.2022.119330.
Underwood R, Tolmeijer E, Wibroe J, Peters E, Mason L. Networks underpinning emotion: a systematic review and synthesis of functional and effective connectivity. Neuroimage. 2021;243:118486. https://doi.org/10.1016/j.neuroimage.2021.118486.
Zhang X, Zhang R, Lv L, Qi X, Shi J, Xie S. Correlation between cognitive deficits and dorsolateral prefrontal cortex functional connectivity in first-episode depression. J Affect Disord. 2022. https://doi.org/10.1016/j.jad.2022.06.024.
Mo Y, Wei Q, Bai T, Zhang T, Lv H, Zhang L. Bifrontal electroconvulsive therapy changed regional homogeneity and functional connectivity of left angular gyrus in major depressive disorder. Psychiatry Res. 2020;294:113461. https://doi.org/10.1016/j.psychres.2020.113461.
Gong J, Wang J, Qiu S, Chen P, Luo Z, Wang J. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl Psychiatry. 2020;10(1):1–13. https://doi.org/10.1038/s41398-020-01036-5.
Chen F, Gong J, Chen G, Chen P, Zhong S, Tang G. Shared and specific characteristics of regional cerebral blood flow and functional connectivity in unmedicated bipolar and major depressive disorders. J Affect Disord. 2022;309:77–84. https://doi.org/10.1016/j.jad.2022.04.099.
Li G, Liu Y, Zheng Y, Wu Y, Li D, Liang X. Multiscale neural modeling of resting-state fMRI reveals executive-limbic malfunction as a core mechanism in major depressive disorder. NeuroImage Clinical. 2021;31:102758. https://doi.org/10.1016/j.nicl.2021.102758.
Hai T, Swansburg R, Kahl CK, Frank H, Stone K, Lemay J-F. Right Superior Frontal Gyrus Cortical Thickness in Pediatric ADHD. J Atten Disord. 2022. https://doi.org/10.1177/10870547221110918.
Morcom AM, Henson RN. Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. J Neurosci. 2018;38(33):7303–13. https://doi.org/10.1523/JNEUROSCI.1701-17.2018.
Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61(4):1213–25. https://doi.org/10.1016/j.neuroimage.2012.04.011.
King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. 2019;22(8):1371–8. https://doi.org/10.1038/s41593-019-0436-x.
Ceravolo L, Frühholz S, Pierce J, Grandjean D, Péron J. Basal ganglia and cerebellum contributions to vocal emotion processing as revealed by high-resolution fMRI. Sci Rep. 2021;11(1):1–15. https://doi.org/10.1038/s41598-021-90222-6.
Habas C. Research note: a resting-state, cerebello-amygdaloid intrinsically connected network. Cerebellum Ataxias. 2018;5(1):1–4. https://doi.org/10.1186/s40673-018-0083-0.
Depping MS, Schmitgen MM, Kubera KM, Wolf RC. Cerebellar contributions to major depression. Front Psych. 2018;9:634. https://doi.org/10.3389/fpsyt.2018.00634.
Auerbach RP, Pagliaccio D, Allison GO, Alqueza KL, Alonso MF. Neural correlates associated with suicide and nonsuicidal self-injury in youth. Biol Psychiat. 2021;89(2):119–33. https://doi.org/10.1016/j.biopsych.2020.06.002.
Arsalidou M, Vijayarajah S, Sharaev M. Basal ganglia lateralization in different types of reward. Brain Imaging Behav. 2020;14(6):2618–46. https://doi.org/10.1007/s11682-019-00215-3.
Wang L, An J, Gao HM, Zhang P, Chen C, Li K, et al. Duloxetine effects on striatal resting-state functional connectivity in patients with major depressive disorder. Hum Brain Mapp. 2019;40(11):3338–46. https://doi.org/10.1002/hbm.24601.
Schaub AC, Kirschner M, Schweinfurth N, Mählmann L, Kettelhack C, Engeli EE, et al. Neural mapping of anhedonia across psychiatric diagnoses: a transdiagnostic neuroimaging analysis. NeuroImage Clinical. 2021;32:102825. https://doi.org/10.1016/j.nicl.2021.102825.
Santarnecchi E, Egiziano E, D’Arista S, Gardi C, Romanella SM, Mencarelli L, et al. Mindfulness-based stress reduction training modulates striatal and cerebellar connectivity. J Neurosci Res. 2021;99(5):1236–52. https://doi.org/10.1002/jnr.24798.
Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K, et al. Consensus paper: towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum. 2017;16(1):203–29. https://doi.org/10.1007/s12311-016-0763-3.
Lu F, Cui Q, Huang X, Li L, Duan X, Chen H. Anomalous intrinsic connectivity within and between visual and auditory networks in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2020;100:109889. https://doi.org/10.1016/j.pnpbp.2020.109889.
Parhizi B, Daliri MR, Behroozi M. Decoding the different states of visual attention using functional and effective connectivity features in fMRI data. Cogn Neurodyn. 2018;12(2):157–70. https://doi.org/10.1007/s11571-017-9461-1.
Liu DY, Ju X, Gao Y, Han JF, Li Z, Hu XW, et al. From molecular to behavior: higher order occipital cortex in major depressive disorder. Cereb Cortex. 2021. https://doi.org/10.1093/cercor/bhab343.
Yan W, Palaniyappan L, Liddle PF, Rangaprakash D, Wei W, Deshpande G. Characterization of hemodynamic alterations in schizophrenia and bipolar disorder and their effect on resting-state fMRI functional connectivity. Schizophr Bull. 2022;48(3):695–711. https://doi.org/10.1093/schbul/sbab140.
Yan W, Rangaprakash D, Deshpande G. Aberrant hemodynamic responses in autism: implications for resting state fMRI functional connectivity studies. NeuroImage Clinical. 2018;19:320–30. https://doi.org/10.1016/j.nicl.2018.04.013.
Wu GR, Colenbier N, Van Den Bossche S, Clauw K, Johri A, Tandon M. rsHRF: a toolbox for resting-state HRF estimation and deconvolution. Neuroimage. 2021;244:118591. https://doi.org/10.1016/j.neuroimage.2021.118591.
Funding
This work was supported by Natural Science Foundation of Hunan Province of China (No. 2019JJ40387), the National Natural Science Foundation of China (No.61972419 and 61672542), the Natural Science Foundation of Hunan Province of China (No. 2020JJ4120), and the Fundamental Research Funds for the Central Universities of Central South University. We thank REST-meta-MDD Consortium for sharing and providing dataset, and we are grateful for resources from the High Performance Computing Center of Central South University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, P., Zhou, X., Xiong, T. et al. Altered Effective Connectivity Among the Cerebellum and Cerebrum in Patients with Major Depressive Disorder Using Multisite Resting-State fMRI. Cerebellum 22, 781–789 (2023). https://doi.org/10.1007/s12311-022-01454-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-022-01454-9