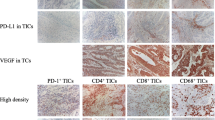
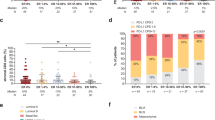
Abstract
The recent successes of new cancer immunotherapy approaches have led to investigate their relevance in the context of the Endometrial Carcinoma (EC). These therapies, that take the tumor-induced immunosuppressive microenvironment into account, target the tumor immune escape, in particular the inhibitory receptors involved in the regulation of the effector T cells’ activity (immune checkpoints). The aim of this study was to identify, in ECs, differences in intergrades immune status that could contribute to the differences in tumor aggressiveness, and could also be used as theranostic tools. The immune status of tumors was assessed by quantitative real-time PCR. We analyzed the expression of specific genes associated to specific leukocytes subpopulations and the expression of reporting genes associated with the tumor escape/resistance. This study highlights significant differences in the EC intergrades immune status especially the tumor-infiltrating cell types and their activation status as well as in the molecular factors produced by the environment. The immune microenvironment of grade 1 ECs hints at a robust tumoricidal milieu while that of higher grades is more evocative of a tolerogenic milieu. This genes-based immunological monitoring of tumors that easily highlights significant intergrade differences relating to the density, composition and functional state of the leukocyte infiltrate, could give solid arguments for choosing the best therapeutic options, especially those targeting immune checkpoints. Moreover it could enable an easy adaptation of individual treatment approaches for each patient.




Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386. https://doi.org/10.1002/ijc.29210
Shim SH, Kim DY, Kim HJ, Lee SW, Park JY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH (2017) Stratification of risk groups according to survival after recurrence in endometrial cancer patients. Medicine (Baltimore) 96(21):e6920. https://doi.org/10.1097/MD.0000000000006920
Tran AQ, Gehrig P (2017) Recent advances in endometrial Cancer. F1000Res 6:81. https://doi.org/10.12688/f1000research.10020.1
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MDM, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L (2013) Mutational landscape and significance across 12 major cancer types. Nature 502(7471):333–339. https://doi.org/10.1038/nature12634
Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, Leary A, Edmondson RJ, Powell ME, Crosbie EJ, Kitchener HC, Mileshkin L, Pollock PM, Smit VT, Creutzberg CL (2015) Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod Pathol 28(6):836–844. https://doi.org/10.1038/modpathol.2015.43
Balkwill FR, Capasso M, Hagemann T (2012) The tumor microenvironment at a glance. J Cell Sci 125 (Pt 23:5591–5596. https://doi.org/10.1242/jcs.116392
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264. https://doi.org/10.1038/nrc3239
Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank CU, Hamid O, Mateus C, Shapira-Frommer R, Kosh M, Zhou H, Ibrahim N, Ebbinghaus S (2015) Ribas a, investigators K- (2015) Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med 372(26):2521–2532. https://doi.org/10.1056/NEJMoa1503093
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, Wolchok JD (2015) Combined Nivolumab and Ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373(1):23–34. https://doi.org/10.1056/NEJMoa1504030
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate I (2015) Nivolumab versus Everolimus in advanced renal-cell carcinoma. N Engl J Med 373(19):1803–1813. https://doi.org/10.1056/NEJMoa1510665
Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA (2016) PD-1 blockade with Pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med 374(26):2542–2552. https://doi.org/10.1056/NEJMoa1603702
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung Cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Manson G, Norwood J, Marabelle A, Kohrt H, Houot R (2016) Biomarkers associated with checkpoint inhibitors) Annals of oncology : official journal of the European society for. Medical Oncology / ESMO 27(7):1199–1206. https://doi.org/10.1093/annonc/mdw181
Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH (2016) Coinhibitory pathways in immunotherapy for Cancer. Annu Rev Immunol 34:539–573. https://doi.org/10.1146/annurev-immunol-032414-112049
Mahoney KM, Rennert PD, Freeman GJ (2015) Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 14(8):561–584. https://doi.org/10.1038/nrd4591
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13(5):273–290. https://doi.org/10.1038/nrclinonc.2016.25
Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK (2017) PD-1 and PD-L1 checkpoint signaling inhibition for Cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 8:561. https://doi.org/10.3389/fphar.2017.00561
Hughes PE, Caenepeel S, Wu LC (2016) Targeted therapy and checkpoint immunotherapy combinations for the treatment of Cancer. Trends Immunol 37(7):462–476. https://doi.org/10.1016/j.it.2016.04.010
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E (2016) Endometrial cancer. Lancet 387(10023):1094–1108. https://doi.org/10.1016/S0140-6736(15)00130-0
Vanderstraeten A, Luyten C, Verbist G, Tuyaerts S, Amant F (2014) Mapping the immunosuppressive environment in uterine tumors: implications for immunotherapy. Cancer Immunol Immunother 63(6):545–557. https://doi.org/10.1007/s00262-014-1537-8
Herzog TJ, Arguello D, Reddy SK, Gatallica Z (2015) PD-1, PD-L1 expression in 1599 gynecological cancers: implications for immunotherapy. Gynecol Oncol 137 (Supplement 1:204–205. https://doi.org/10.1016/j.ygyno.2015.01.514
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC, D'Andrea AD, Wu CJ, Matulonis UA, Konstantinopoulos PA (2015) Association of Polymerase e-mutated and microsatellite-instable endometrial cancers with Neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1(9):1319–1323. https://doi.org/10.1001/jamaoncol.2015.2151
Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, Carrigan M, Saraf S, Chen M, Soria JC (2017) Safety and antitumor activity of Pembrolizumab in advanced programmed death ligand 1-positive endometrial Cancer: results from the KEYNOTE-028 study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 35(22):2535–2541. https://doi.org/10.1200/JCO.2017.72.5952
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA, Jr. (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372 (26):2509–2520. doi:https://doi.org/10.1056/NEJMoa1500596
Kurman RJ, International Agency for Research on Cancer, World Health Organization (2014) WHO classification of tumours of female reproductive organs. World Health Organization classification of tumours, 4th edn. International Agency for Research on Cancer, Lyon
Creasman W (2009) Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet 105(2):109. https://doi.org/10.1016/j.ijgo.2009.02.010
Mocellin S, Provenzano M, Rossi CR, Pilati P, Nitti D, Lise M (2003) Use of quantitative real-time PCR to determine immune cell density and cytokine gene profile in the tumor microenvironment. J Immunol Methods 280(1–2):1–11
Danaher P, Warren S, Dennis L, D'Amico L, White A, Disis ML, Geller MA, Odunsi K, Beechem J, Fling SP (2017) Gene expression markers of tumor infiltrating leukocytes. Journal for immunotherapy of cancer 5:18. https://doi.org/10.1186/s40425-017-0215-8
Cernadas M, Lu J, Watts G, Brenner MB (2009) CD1a expression defines an interleukin-12 producing population of human dendritic cells. Clin Exp Immunol 155(3):523–533. https://doi.org/10.1111/j.1365-2249.2008.03853.x
Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS (2010) CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-kappa B. J Immunol 184(10):5571–5581. https://doi.org/10.4049/jimmunol.0803494
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19(11):1423–1437. https://doi.org/10.1038/nm.3394
Mantovani A, Barajon I, Garlanda C (2018) IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol Rev 281(1):57–61. https://doi.org/10.1111/imr.12614
Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, Fujii H (2008) CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer 122(10):2286–2293. https://doi.org/10.1002/ijc.23392
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH (2006) Pages F (2006) type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964
Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL Jr (2012) Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res 72(5):1070–1080. https://doi.org/10.1158/0008-5472.CAN-11-3218
Manaster I, Mandelboim O (2010) The unique properties of uterine NK cells. Am J Reprod Immunol 63(6):434–444. https://doi.org/10.1111/j.1600-0897.2009.00794.x
Vanderstraeten A, Tuyaerts S, Amant F (2015) The immune system in the normal endometrium and implications for endometrial cancer development. J Reprod Immunol 109:7–16. https://doi.org/10.1016/j.jri.2014.12.006
Jago CB, Yates J, Camara NO, Lechler RI, Lombardi G (2004) Differential expression of CTLA-4 among T cell subsets. Clin Exp Immunol 136(3):463–471. https://doi.org/10.1111/j.1365-2249.2004.02478.x
Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE, Chen PL, Hwu P, Allison JP, Futreal A, Wargo JA, Sharma P (2016) Loss of IFN-gamma pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 167(2):397–404 e399. https://doi.org/10.1016/j.cell.2016.08.069
Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, Logothetis C, Sharma P (2008) CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 105(39):14987–14992. https://doi.org/10.1073/pnas.0806075105
Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A (2016) Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 375(9):819–829. https://doi.org/10.1056/NEJMoa1604958
Shi L, Chen S, Yang L, Li Y (2013) The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol 6(1):74. https://doi.org/10.1186/1756-8722-6-74
Taylor NA, Vick SC, Iglesia MD, Brickey WJ, Midkiff BR, McKinnon KP, Reisdorf S, Anders CK, Carey LA, Parker JS, Perou CM, Vincent BG, Serody JS (2017) Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J Clin Invest 127(9):3472–3483. https://doi.org/10.1172/JCI90499
Anderson AC, Joller N, Kuchroo VK (2016) Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44(5):989–1004. https://doi.org/10.1016/j.immuni.2016.05.001
Ferris RL, Lu B, Kane LP (2014) Too much of a good thing? Tim-3 and TCR signaling in T cell exhaustion. J Immunol 193(4):1525–1530. https://doi.org/10.4049/jimmunol.1400557
Banerjee H, Kane LP (2018) Immune regulation by Tim-3. F1000Res 7:316. https://doi.org/10.12688/f1000research.13446.1
Zhou X, Sun L, Jing D, Xu G, Zhang J, Lin L, Zhao J, Yao Z, Lin H (2018) Galectin-9 expression predicts favorable clinical outcome in solid tumors: a systematic review and meta-analysis. Front Physiol 9:452. https://doi.org/10.3389/fphys.2018.00452
Dai SY, Nakagawa R, Itoh A, Murakami H, Kashio Y, Abe H, Katoh S, Kontani K, Kihara M, Zhang SL, Hata T, Nakamura T, Yamauchi A, Hirashima M (2005) Galectin-9 induces maturation of human monocyte-derived dendritic cells. J Immunol 175(5):2974–2981
Nagahara K, Arikawa T, Oomizu S, Kontani K, Nobumoto A, Tateno H, Watanabe K, Niki T, Katoh S, Miyake M, Nagahata S, Hirabayashi J, Kuchroo VK, Yamauchi A, Hirashima M (2008) Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol 181(11):7660–7669
Kadowaki T, Arikawa T, Shinonaga R, Oomizu S, Inagawa H, Soma G, Niki T, Hirashima M (2012) Galectin-9 signaling prolongs survival in murine lung-cancer by inducing macrophages to differentiate into plasmacytoid dendritic cell-like macrophages. Clin Immunol 142(3):296–307. https://doi.org/10.1016/j.clim.2011.11.006
Acknowledgements
We are grateful to Professor Jean-François Michiels from the Central Anatomy Laboratory of Nice Pasteur University Hospital who provided expertise that greatly assisted the cytopathological examination of tumors.
Funding
This study was supported in part by the Centre Hospitalier Universitaire (CHU) de Nice (Appel à Projet Interne 2015), the Centre National de la Recherche Scientifique (CNRS) and the Institut National de la Santé et de la Recherche médicale (INSERM).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Heidy Schmid-Antomarchi, Annie Schmid-Alliana.
Performed the experiments: Julie Antomarchi, Charlotte Cohen, Babou Karimdjee-Soilihi.
Analyzed the data: Julie Antomarchi, Charlotte Cohen, Annie Schmid-Alliana, Heidy Schmid-Antomarchi.
Contributed reagents/materials/analysis tools: Jérome Delotte, Anne Chevallier, Mélanie Ngo-Mai, Damien Ambrosetti.
Wrote the paper: Heidy Schmid-Antomarchi, Annie Schmid-Alliana, Julie Antomarchi, Charlotte Cohen .
Corresponding author
Ethics declarations
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standarts.
For this study formal consent is not requiered.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 153 kb)
Rights and permissions
About this article
Cite this article
Antomarchi, J., Ambrosetti, D., Cohen, C. et al. Immunosuppressive Tumor Microenvironment Status and Histological Grading of Endometrial Carcinoma. Cancer Microenvironment 12, 169–179 (2019). https://doi.org/10.1007/s12307-019-00225-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-019-00225-1