Abstract
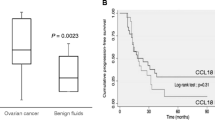
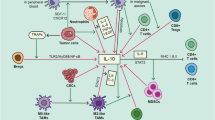
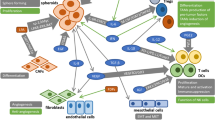
Ovarian cancer (OC) ascites is an inflammatory and immunosuppressive tumor environment characterized by the presence of various cytokines, chemokines and growth factors. The presence of high concentrations of these cytokines/chemokines in ascites is associated with a more aggressive tumor phenotype. IL-10 is an immunosuppressive cytokine for which high expression has been associated with poor prognosis in some cancers. However, its role on OC tumor cells has not been explored. Therefore, the aim of the current study was to elucidate the role of ascites IL-10 on the proliferation, migration and survival of OC cell lines. Here, we show that IL-10 levels are markedly increased in patients with advanced serous OC ascites relative to serous stage I/II ascites and peritoneal effusions from women with benign conditions. Ascites and IL-10 dose-dependently enhanced the proliferation and migration of OC cell lines CaOV3 and OVCAR3 but had no effect on cell survival. IL-10 levels in ascites positively correlated with the ability of ascites to promote cell migration but not proliferation. Depletion of IL-10 from ascites markedly inhibited ascites-induced OC cell migration but was not crucial for ascites-mediated cell proliferation. Taken together, our findings establish an important role for IL-10, as a component of ascites, in the migration of tumor cells.





Similar content being viewed by others
References
Bast RC, Hennessy B, Mills GB (2009) The biology of ovarian cancer: new opportunities for translation. Nat Rev Cancer 9:415–428
Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, Xu X, Hamilton TC (2004) Focus on epithelial ovarian cancer. Cancer Cell 5:19–24
Shen-Gunther J, Mannel RS (2002) Ascites as a predictor of ovarian malignancy. Gynecol Oncol 87:77–83
Ayhan A, Gultekin M, Taskiran C, Dursun P, Firat P, Bozdag G, Celik NY, Yuce K (2007) Ascites and epithelial ovarian cancers: a reappraisal with respect to different aspects. Int J Gynecol Cancer 17:68–75
Ayantunde AA, Parsons SL (2007) Pattern and prognostic factors in patients with malignant ascites: a retrospective study. Ann Oncol 18:945–949
Matte I, Lane D, Laplante C, Rancourt C, Piché A (2012) Profiling of cytokines in human epithelial ovarian cancer ascites. Am J Cancer Res 2:566–580
Lane D, Matte I, Rancourt C, Piché A (2011) Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer 11:210
Giuntoli RL, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M (2009) Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res 29:2875–2884
Maccio A, Madeddu C (2012) Inflammation and ovarian cancer. Cytokine 58:133–147
Germano G, Allavena P, Mantovani A (2008) Cytokines as a key component of cancer-related inflammation. Cytokine 43:374–379
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Lane D, Robert V, Grondin R, Rancourt C, Piché A (2007) Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt in human ovarian carcinoma cells. Int J Cancer 121:1227–1237
Lane D, Goncharenko-Khaider N, Rancourt C, Piché A (2010) Ovarian cancer ascites protects from TRAIL-induced cell death through αvβ5 integrin-mediated focal adhesion kinase and Akt activation. Oncogene 29:3519–3531
Goncharenko-Khaider N, Matte I, Lane D, Rancourt C, Piché A (2012) Ovarian cancer ascites increase Mcl-1 expression in tumor cells through ERK1/2-Elk-1 signaling to attenuate TRAIL-induced apoptosis. Mol Cancer 11:84
Puiffe ML, Le Page C, Filali-Mouhim A, Zietarska M, Ouellet V, Tonin PN, Chevrette M, Provencher DM, Mes-Masson AM (2007) Characterization of ovarian cancer ascites on cell invasion, proliferation, spheroid formation, and gene expression in an in vitro model of epithelial ovarian cancer. Neoplasia 9:820–829
Matte I, Lane D, Laplante C, Garde-Granger P, Rancourt C, Piché A (2015) Ovarian cancer ascites enhance the migration of patient-derived peritoneal mesothelial cells via cMet pathway through HGF-dependent and –independent mechanisms. Int J Cancer 137:289–298
Mannino MH, Zhu Z, Xiao H, Bai Q, Wakefield MR, Fang Y (2015) The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 367:103–107
Jankovic D, Kugler DG, Sher A (2010) IL-10 production by CD4+ effector T cells: a mechanism for self-regulation. Mucosal Immunol 3:239–246
Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A (2007) Conventional T-bet(+) Foxp3(−) Th1 cells are the major source of host protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204:273–283
Maynard CL, Weaver CT (2008) Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev 226:219–233
Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M (2004) The multifaceted relationship between IL-10 and adaptive immunity: putting together the pieces of a puzzle. Cytokine Growth Factor Rev 15:61–76
Umetsu SE, Winandy S (2009) Ikaros is a regulator of Il10 expression in CD4+ T cells. J Immunol 183:5518–5525
Li C, Li H, Jiang K, Li J, Gai X (2014) TLR4 signaling pathway in mouse Lewis lung cancer cells promotes the expression of TGF-β1 and IL-10 and tumor cells migration. Biomed Mater Eng 24:869–875
Gastl GA, Abrams JS, Nanus DM, Oosterkamp R, Silver J, Liu F, Chen M, Albino AP, Bander NH (1993) Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer 55:96–101
Krüger-Krasagakes S, Krasagakis K, Garbe C, Schmitt E, Hüls C, Blankenstein T, Diamantstein T (1994) Expression of interleukin 10 in human melanoma. Br J Cancer 70:1182–1185
Boyano MD, Garcia-Vazquez MD, Lopez-Michelena T, Gardeazabal J, Bilbao J, Canavate M, Galdeano AG, Izu R, Diaz-Ramon L, Raton JA, Diaz-Perez JL (2000) Soluble interleukin-2 receptor, intercellular adhesion molecule-1 and interleukin-10 serum levels in patients with melanoma. Br J Cancer 83:847–852
Chen Q, Daniel V, Maher DW, Hersey P (1994) Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int J Cancer 56:755–760
Nemunaitis J, Fong T, Shabe P, Martineau D, Ando D (2001) Comparison of serum interleukin-10 (IL-10) levels between normal volunteers and patients with advanced melanoma. Cancer Investig 19:239–247
Sato T, McCue P, Masuoka K, Salwen S, Lattime EC, Mastrangelo MJ, Berd D (1996) Interleukin 10 production by human melanoma. Clin Cancer Res 2:1383–1390
Boulland ML, Meignin V, Leroy-Viard K, Copie-Bergman C, Brière J, Touitou R, Kanavaros P, Gaulard P (1998) Human interleukin-10 expression in T/natural killer-cell lymphomas: association with anaplastic large cell lymphomas and nasal natural killer-cell lymphomas. Am J Pathol 153:1229–1237
Nowark M, Glowacka E, Szpakowski M, Szyllo K, Malinowski A, Kulig A, Tchorzewski H, Wilczynski J (2010) Proinflammatory and immunosuppressive serum, ascites and cyst fluid cytokines in patients with early and advanced ovarian cancer and benign ovarian tumors. Neuro Endocrinol Lett 31:375–383
Gotlieb WH, Abrams JS, Watson JM, Velu TJ, Berek JS, Martinez-Maza O (1992) Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine 4:385–390
Mustea A, Konsgen D, Braicu EI, Pirvulescu C, Sun P, Sofroni D, Lichtenegger W, Sehouli J (2006) Expression of IL-10 in patients with ovarian carcinoma. Anticancer Res 26:1715–1718
Berger S, Siegert A, Denkert C, Kobel M, Hauptmann S (2001) Interleukin-10 in serous ovarian carcinoma cell lines. Cancer Immunol Immunother 50:328–333
Zhou J, Ye F, Chen H, Lv W, Gan N (2007) The expression of interleukin-10 in patients with primary ovarian epithelial carcinoma and in ovarian carcinoma cell lines. J Int Med Res 35:290–300
Brencicova E, Jagger AL, Evans HG, Georgouli M, Laios AH, Montalto S, Mehra G, Spencer J, Ahmed AA, Raju-Kankipati S, Taams LS, Diebold SS (2017) Interleukin-10 and prostaglandin E2 have complementary but distinct suppressive effects on toll-like receptor-mediated dendritic cell activation in ovarian carcinoma. PLoS One 12:e0175712
Lamichhane P, Karyampudi L, Shreeder B, Krempski J, Bahr D, Daum J, Kalli KR, Goode EL, Block MS, Cannon MJ, Knutson KL (2017) IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res 77:6667–6678
Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD (2000) FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249–256
Lane D, Matte I, Laplante C, Garde-Granger P, Carignan A, Bessette P, Rancourt C, Piché A (2016) CCL18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol Cancer 15:58
Wu L, Deng Z, Peng Y, Han L, Liu J, Wang L, Li B, Zhao J, Jiao S, Wei H (2017) Ascites-derived IL-6 and IL-10 synergistically expand CD14+HLA-DR−/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget 8:76843–76856
Kohno T, Mizukami H, Suzuki M, Saga Y, Takei Y, Shimpo M, Matsushita T, Okada T, Hanazono Y, Kume A, Sato I, Ozawa K (2003) Interleukin-10-mediated inhibition of angiogenesis and tumor growth in mice bearing VEGF-producing ovarian cancer. Cancer Res 63:5091–5094
Deying W, Feng G, Shumei L, Hui Z, Ming L, Hongqing W (2017) CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci Rep 37:BSR20160470
Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN (2011) Lysophosphatidic acid stimulates the proliferation of ovarian Cancer cells via the gep proto-oncogene Gα(12). Genes Cancer 2:563–575
Wang Y, Li L, Guo X, Jin X, Sun W, Zhang X, Xu RC (2012) Interleukin-6 signaling regulates anchorage-independent growth, proliferation, adhesion and invasion in human ovarian cancer cells. Cytokine 59:228–236
Yousif NG (2014) Fibronectin promotes migration and invasion of ovarian cancer cells through up-regulation of FAK-PI3K/Akt pathway. Cell Biol Int 38:85–91
Bian D, Su S, Mahanivong C, Cheng RK, Han Q, Pan ZK, Sun P, Huang S (2004) Lysophosphatidic acid stimulates ovarian cancer cell migration via a Ras-MEK kinase 1 pathway. Cancer Res 64:4209–4217
Lo CW, Chen MW, Hsiao M, Wang S, Chen CA, Hsiao SM, Chang JS, Lai TC, Rose-John S, Kuo ML, Wei LH (2011) IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res 71:424–434
Furukama S, Soeda S, Kiko Y, Suzuki O, Hashimoto Y, Watanabe T, Nishiyama H, Tasaki K, Hojo H, Abe M, Fujimori K (2013) MCP-1 promotes invasion and adhesion of human ovarian cancer cells. Anticancer Res 33:4785–4790
Sung WW, Wang YC, Lin PL, Cheng YW, Chen CY, Wu TC, Lee H (2013) IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Cancer Res 19:4092–4103
Chen XD, Tang SX, Zhang JH, Zhang LT, Wang YW (2017) CIP2A, an oncoprotein, is associated with cell proliferation, invasion and migration in laryngeal carcinoma cells. Oncol Rep 38:1005–1012
Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E, Wolk K, Geginat J (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21:331–344
Kipps E, Tan DS, Kaye SB (2013) Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev 13:273–282
Rabinovich A, Medina L, Piura B, Huleihel M (2010) Expression of IL-10 in human normal and cancerous ovarian tissues and cells. Eur Cytokine Netw 21:122–128
Yao V, Platell C, Hall JC (2004) Peritoneal mesothelial cells produce inflammatory related cytokines. ANZ J Surg 74:997–1002
Alldredge J, Flies D, Higuchi T, Ma T, Adams SF (2014) Impaired interleukin-10 signaling and ovarian cancer growth in the peritoneal cavity. J Clin Oncol 32(15 suppl):e22094–e22094
Acknowledgements
This work was supported by funds from the Canadian Institute for Health Research (MOP-244194-CPT-CFDA-48852), the Centre de Recherche Clinique Étienne-Lebel du Centre Hospitalier Universitaire de Sherbrooke and the Université de Sherbrooke. Tumor banking were supported by the Banque de tissus et données of the Réseau de recherche sur le cancer of the Fond de recherche du Québec en Santé (FRQS) and Cancer de l’Ovaire Canada, associated with the Canadian Tumor Repository Network (CTRNet). The tissue bank provided malignant ascites and peritoneal fluids.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PPT 265 kb)
Rights and permissions
About this article
Cite this article
Lane, D., Matte, I., Garde-Granger, P. et al. Ascites IL-10 Promotes Ovarian Cancer Cell Migration. Cancer Microenvironment 11, 115–124 (2018). https://doi.org/10.1007/s12307-018-0215-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12307-018-0215-3