Abstract
The purpose of this systematic review was to analyze the current use of adipose-derived mesenchymal stem cells (ADMSCs) and present the available evidence on their therapeutic potential in the treatment of ankle orthopedic issues, evaluating the applications and results. A literature search of PubMed, Google Scholar, EMBASE and Cochrane Library database was performed. The review was conducted following PRISMA guidelines. Risk of bias assessment was conducted through the Methodological Index for Non-Randomized Studies (MINORS) criteria. Initial search results yielded 4348 articles. A total of 8 articles were included in the review process. No clinical evidence has demonstrated the effectiveness of one isolation method over the other, but nonenzymatic mechanical method has more advantages. In all studies included significant clinical outcomes improvement were recorded in patients affected by osteochondral lesion and osteoarthritis of ankle. All studies performed a concomitant procedure. No serious complications were reported. ADMSC injection, especially through the nonenzymatic mechanical methods, looks to be simple and promising treatment for osteochondral lesions and osteoarthritis of the ankle, with no severe complications. The current scarcity of studies and their low-quality level preclude definitive conclusions presently.
Level of evidence
III.
Similar content being viewed by others
Introduction
Mesenchymal stem cells (MSCs) have been isolated from bone marrow, periosteum, umbilical cord blood, dermis, infrapatellar fat pad, adipose tissue, synovium, skeletal muscles, and deciduous teeth [1]. These cells are multi-potent stem cells capable of differentiating into cells of connective tissue lineages. It is now commonly accepted that their action mechanism is mainly due to MSCs paracrine expression of a variety of bioactive factors acting with immunomodulatory and trophic fashion. Indeed, the patient's own resident stem cells construct the new tissue, stimulated by the bioactive factors secreted by the exogenously supplied MSCs [2]. The MSCs therefore may provide chondrogenic and chondroprotective capacity to arthritic joints [1, 3, 4]. For these reasons, MSCs have attracted attention as an ortho-biologic cellular therapy in regenerative medicine [5,6,7,8].
Although several sources from adult progenitor cells have been reported, in the last decade, adipose-derived mesenchymal stem cells (ADMSCs) have been recognized as an alternative source of stromal cells [9, 10]. Some studies [11,12,13] showed that ADMSCs have a chondrogenic potential similar to bone marrow derived MSCs, and moreover are easier to be obtained. As a matter of fact, subcutaneous stores in the infrapatellar fat pad and buttocks/flank allow for a less invasive harvesting process with lower donor site morbidity and lesser complications than the other stromal cells harvesting. Finally, lipoaspirate has been demonstrated to result in higher progenitor cell yields than bone marrow aspirates [11,12,13,14,15].
According to the isolation methods, three different categories of adipose-derived therapies can be identified: Adipose-derived stem cells (ADSCs), stromal vascular fraction (SVF), and micronized adipose tissue (MAT). The term ADSCs should be used when referring to MSCs isolated from adipose tissue and expanded in culture [16]. SVF typically requires centrifugation and collagenase enzymatic digestion procedures, where the cells are re-leased from their collagen matrix [17]. Mechanical separation of adipose tissue without using collagenase releases the cells from lipoaspirate, producing “micronized fat” (MAT) through minimal manipulation [18].
Despite the growing research on the role of ADMSCs therapy in osteoarthritis and cartilage repair, the scientific production has been less focused on the ankle.
The purpose of this systematic review was to analyze the current use in literature of ADMSCs in humans and presents the available evidence on their therapeutic potential in the treatment of ankle orthopedic issues, evaluating their applications and results.
Materials and methods
Search strategy
A review of the literature concerning the clinical applications of ADMSCs in ankle orthopedic pathologies was conducted independently by 2 of the authors (AA and EA) using PubMed, Google Scholar, EMBASE and Cochrane Library database on March 1, 2023. The search terms used were: “adipose derived stem cells”, “ankle”, “talus”. Field codes were used for database searches and each database was searched using the specific retrieve terms, and Medical Subject Headings (MeSH). The complete retrieve strategies were the following: ("ankle"[MeSH Terms] OR "ankle"[All Fields] OR "ankle joint"[MeSH Terms] OR ("ankle"[All Fields] AND "joint"[All Fields]) OR "ankle joint"[All Fields] OR "ankles"[All Fields] OR "ankle s"[All Fields] OR ("talus"[MeSH Terms] OR "talus"[All Fields])) AND (("adipose tissue"[MeSH Terms] OR ("adipose"[All Fields] AND "tissue"[All Fields]) OR "adipose tissue"[All Fields] OR "adipose"[All Fields] OR "adiposities"[All Fields] OR "adiposity"[MeSH Terms] OR "adiposity"[All Fields]) AND ("analogs and derivatives"[MeSH Subheading] OR ("analogs"[All Fields] AND "derivatives"[All Fields]) OR "analogs and derivatives"[All Fields] OR "derivatives"[All Fields] OR "de-rivable"[All Fields] OR "derivant"[All Fields] OR "derivants"[All Fields] OR "deri-vate"[All Fields] OR "derivated"[All Fields] OR "derivates"[All Fields] OR "deriva-tion"[All Fields] OR "derivations"[All Fields] OR "derivative"[All Fields] OR "derive"[All Fields] OR "derived"[All Fields] OR "derives"[All Fields] OR "deriving"[All Fields]) AND ("stem cells"[MeSH Terms] OR ("stem"[All Fields] AND "cells"[All Fields]) OR "stem cells"[All Fields])).
Reference lists of all included publications were checked for potential studies.
Selection criteria
The PRISMA (Preferred Reporting Item for Systematic Reviews and Meta-Analyses) guidelines were followed, and a flowchart was used to summarize the selection procedure of the reviewed studies [19].
Inclusion criteria were determined and agreed upon between the reviewers. The inclusion criterium was the use of ADSCs in humans applied to bony orthopedic diseases of the ankle such as osteoarthritis and osteochondral lesions.
Exclusion criteria were non-English publications, review and meta-analyses articles, expert opinions and letter to Editor, animal studies and in vitro studies, participants under 18 years old, rheumatic diseases and septic ankle, the absence of clinical evaluation outcomes scores.
After duplicates removal, title and abstracts of all articles were screened for eligibility independently by 2 reviewers (AA and EA) and the papers of interest were selected for the full text. At full-text review, agreement of 2 reviewers was needed for study inclusion or exclusion. Disputes regarding inclusion of an article were resolved from the senior author (CF).
Data abstraction and quality assessment
The included studies were analyzed by two reviewers to collect the following data according to PICO question (participants, intervention, comparisons, and outcomes):
-
Authors, year of publication, study type and level of evidence (LOE).
-
Participants: number of ankles, patients demographic characteristics (age, gender) and mean of follow up.
-
Intervention: pathology, isolation methods, clinical applications, concomitant procedures.
-
Comparisons: differences of clinical outcomes before and after the use of ADSCs.
-
Outcomes: clinical outcomes through the PROMs, such as American Orthopaedic Foot and Ankle Society's (AOFAS), Foot and Ankle Outcome Score (FAOS), Foot and Ankle Disability Index (FADI), Tegner score and Visual analogue scale (VAS), and complications.
Data collection was performed using Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA) for Windows 11.
Quality assessment of included studies was performed by two reviewers (A.A. and E.A.) independently using the Methodological index for non-randomized studies (MINORS) score [20].
Data analysis
Information retrieved from the studies was reported with the use of descriptive statistics. Continuous variables were reported as mean value and standard deviation or range.
Results
Study selection
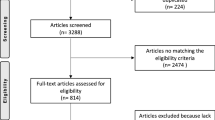
The literature search yielded 4348 articles from Database search engine. After removing duplicates and reviewing all studies according to excluding criteria, 8 articles were identified for full-text review. After this evaluation, all 8 studies met the inclusion criteria and were included in the qualitative synthesis. The selection review process is summarized in Fig. 1.
All articles included were published between 2013 and 2021.
Quality of evidence
The methodological quality assessment, as measured by the MINORS score, is summarized in Table 1. We considered the 8 items of MINORS score for non-comparative study of the eligible papers and the 12 items for comparative study design. The mean MINORS score was 7.3 for non-comparative study and 16.8 for comparative study.
Population data
Patients’ characteristics are reported in Table 2. The total number of included ankles was 167. Six papers reported the gender distribution: in total 64 females (38%) and 79 males (47%) were included. When reported, the patient’s mean age was 49.2 ± 15.1 years (range 42–56.8). Mean follow-up (FU) was 21.4 months.
Isolation method and clinical applications
The ADSCs isolated from adipose tissue and expanded in culture was utilized by only one study [21], moreover they were the lonely that injected ADSCs into the ankle joint through ultrasound guidance 3 times 6 months apart. The remaining studies performed injections on the same day of the arthroscopy after stem cell preparation. Four studies performed intra-articular ankle arthroscopic injection of autologous SVF [22,23,24,25], while the autologous MAT was prepared by 3 studies, 2 of them injected it into the ankle joint through arthroscopic fashion [26, 27], and one performed a closed intra-articular injection [28] (Table 3).
Osteochondral lesions
Four studies [21, 24, 25, 27] dealt with osteochondral lesion of the talus (OCLT), see Table 3. Freitag et al. [21] conducted a case report where the 42 years old patient underwent to prior arthroscopic excision and curettage of a focal OCLT and sequentially 3 times intraarticular ultrasound-guided injection of autologous ADMSCs. FADI score showed significant improvement in pre- to postoperative time (p < 0.05). Moreover, MRI with additional T2 mapping techniques showed successful regeneration of hyaline-like cartilage.
In a case report by D'Ambrosi et al. [27] the AOFAS and VAS score recorded a significant improvement before and after intervention (p < 0.05).
Kim et al. [24] showed how clinical (AOFAS, VAS and Tegner score) and MRI outcomes after an SVF injection with marrow stimulation improved significantly from pre- to postoperative period (p < 0.05) and compared it with marrow stimulation alone.
Kim et al. [25] reported significant improvement in clinical outcomes, including AOFAS, VAS and Tegner score (p < 0.05), in patients over 50 years old with OCLT that had SVF injection with marrow stimulation. Moreover, the outcomes of this group were better compared to those of marrow stimulation alone, especially when the lesion size was larger than 109 mm2 or a subchondral cyst existed.
Osteoarthritis
Post-traumatic osteoarthritis (PTA) was considered in four articles [22, 23, 26, 28] (Table 3). Shimozono et al. [26] divided PTA patients into 2 groups considering Kellgren–Lawrence (KL) classification, 8 patients were collected in grade 3 and 11 cases in grade 4. The outcomes, including FAOS and VAS, showed a significant improvement before and after intervention in all scores, but no significant change was noted for the FAOS subscales of daily activities and symptoms. The overall FAOS score demonstrated a more significant improvement in pre- to postoperative change for KL grade 3 group than KL grade 4 group (p = 0.048).
Natali et al. [28] included 3 patients in KL grade 1, 15 in grade 2 and 13 cases in grade 3. A statistically significant improvement from basal evaluation to the 6, 12-, and 24-month FU was observed for AOFAS, FADI and VAS, whereas a statistically significant worsening from the 12-month to the 24-month FU was recorded.
In 2016, Kim et al. [22, 23] conducted 2 comparative studies. In one paper ADMSC injection with marrow stimulation was compared to marrow stimulation alone in patients with varus ankle osteoarthritis who have undergone lateral sliding calcaneal osteotomy [23]. The other one compared ADMSC injection with marrow stimulation to marrow stimulation alone in patients with varus ankle osteoarthritis treated with supramalleolar osteotomy [22]. The clinical and second-look arthroscopic outcomes of ADMSC injection with marrow stimulation were better related to those of marrow stimulation alone in patients with varus ankle osteoarthritis treated with bony associated procedures.
Concomitant procedure
In 5 papers the authors performed concomitant bone marrow stimulation through arthroscopic microfractures [22,23,24,25, 27], in one article a prior arthroscopic excision and curettage of a focal OCLT was made [21]. Other concomitant procedures were summarized in Table 3. Moreover, 2 studies performed bony procedures: lateral sliding calcaneal osteotomy [23] and supramalleolar osteotomy [22] to treat medial ankle osteoarthritis and varus deformity, associated to bone marrow stimulation and intra-articular ankle arthroscopic injection of autologous SVF.
Complications
No severe side effects were recorded from all studies considering the injection site or the donor site. Natali et al. [28] reported in 5 patients (16%) transitory intra-articular burning sensation after the injection or mild articular pain for a few days. Similar symptoms were recorded by Freitag et al. [21].
Discussion
This systematic review assessed the current literature on the clinical applications and results of ADMSCs in bony orthopaedic diseases of the ankle. Although the literature concerning the knee application of ADMSCs is wide, this paper represents the first systematic review concerning the application of ADMSCs on ankle joint.
Isolation methods and clinical applications
ADSCs expansion step is essential to generate sufficient cell numbers and requires among 24–48 h of incubation [16]. Thus, ADSCs culture present some drawbacks: require a two-stage procedure before administration, are expensive to produce because requiring competent staff and specific laboratory equipment and require a regulatory approval. Moreover, the delivery of ADSCs alone is not sufficient to regenerate damaged cartilage, but if they are incorporated in biomaterial scaffolds with cytokine growth factors, led to a significant increase of proliferation cells and chondrogenic marker expression [29, 30].
Differently, SVF or MAT isolation require a one-step procedure, and are relatively cost saving, but SVF isolation at the point of care for immediate clinical administration has to comply with strict regulatory requirements [17]. On the other hand, MAT method generally is not associated with expensive equipment and can be readily used without regulatory issues of enzymatic manipulation and cell expansion [9, 10, 31, 32]. Additionally, MAT preserves the cell and tissue microarchitecture of adipose tissue and includes high numbers of pericytes cells with an intact functional extracellular matrix [18].
At once, however, no clinical evidence has demonstrated the effectiveness of one system over the others [33, 34] and the current literature is poor about comparison of the various available formulations.
As regards the administration methods and considering the same outcomes, the studies that carry out ultrasound-guided or closed administration reported some complications; however, this finding may be biased by the fact that other studies did not pay attention to or record minor complications.
Osteochondral lesions
Osteochondral lesions seem to better respond to MAT injection than marrow stimulation alone, even in patients over 50 and in large lesions [24, 25].
Generally, significant improvement was recorded to clinical outcomes following cell injection. In the case report by Freitag [21] the patient reported persistent limitation in sporting pursuits and recreational activity, although the T2 mapping MRI showed successful regeneration of hyaline-like cartilage.
Osteoarthritis
It is interesting to note that in one study [26] although the improvement of all outcomes, the AOFAS subscales of daily activities and symptoms did not record significant change. A possible explanation could be that, although MAT can improve patients’ pain, the improvement is not enough to allow them to return to their daily activities. Indeed, MAT injection improved the VAS at 6 months but an increasing in VAS was observed at final FU. Initial symptoms improvement followed by long-term gradual worsening may suggest that MAT therapy provides significant, but gradually decreasing, pain relief in ankle osteoarthritis.
A transient improvement was also observed by Natali et al. [28], who showed a significant worsening from the 12-month to the 24-month FU visit.
Hence, ADMCS therapy may represent a non-surgical option to treat degenerative joint ankle disease in order to postpone invasive procedures especially in younger patients.
Concomitant procedure
Few prospective studies evaluated the benefit of ADMSC in isolated injections. Many studies observed that good outcomes were recorded when axis realignment of a varus deformity was performed [35, 36]. Therefore, it is difficult to determine the therapeutic effect of regenerative medicine when it is associated with bone procedures. Future studies could compare ADMSC injection alone to cell injection associated with bone procedures, in order to verify whether the effect is synergistic or indifferent.
Complications
The studies included in this review reported no serious complications; however, in the literature the most common complications concern the donor site, such as infection and pain. But these were lower than traditional Bone Marrow-MSC harvesting [8].
Limitation
A great limitation can be addressed to the type of studies included, with no randomized double-blinded trials or comparative studies, leading to a lack of a control to confirm the efficacy of ADMSCs. The quality of these studies was extremely poor: notably, four out of eight studies [22,23,24,25] were conducted by the same research team, and two studies [21, 27] are a case report.
Furthermore, in many studies ADMSCs injection was performed in association with other intraarticular injections or surgical procedures, such as debridement, marrow stimulations, bony procedures. Therefore, any clinical results are unable to be attributable solely to the ADSCs injection.
Conclusion
Based on the current literature ADSC injection, especially through the nonenzymatic mechanical methods, looks to be simple and promising treatment, without severe complications, for osteochondral lesions and osteoarthritis of the ankle. The current scarcity of studies and their limited level of evidence preclude definitive conclusions presently. Nonetheless, the encouraging outcomes should stimulate further high-level trial studies in this field.
Change history
06 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12306-023-00809-7
References
Arinzeh TL (2005) Mesenchymal stem cells for bone repair: preclinical studies and potential orthopedic applications. Foot Ankle Clinics 10:651–665. https://doi.org/10.1016/j.fcl.2005.06.004
Caplan AI (2017) Mesenchymal stem cells: time to change the name! Stem Cells Transl Med 6:1445–1451. https://doi.org/10.1002/sctm.17-0051
Barry FP, Murphy JM (2004) Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol 36:568–584. https://doi.org/10.1016/j.biocel.2003.11.001
Teti G, Cavallo C, Grigolo B et al (2012) Ultrastructural analysis of human bone marrow mesenchymal stem cells during in vitro osteogenesis and chondrogenesis. Microsc Res Tech 75:596–604. https://doi.org/10.1002/jemt.21096
Buda R, Vannini F, Cavallo M et al (2013) One-step arthroscopic technique for the treatment of osteochondral lesions of the knee with bone-marrow-derived cells: three years results. Musculoskelet Surg 97:145–151. https://doi.org/10.1007/s12306-013-0242-7
Cavallo C, Desando G, Cattini L et al (2013) Bone marrow concentrated cell transplantation: rationale for its use in the treatment of human osteochondral lesions. J Biol Regul Homeost Agents 27:165–175
Melick G, Hayman N, Landsman AS (2018) Mesenchymal stem cell applications for joints in the foot and ankle. Clin Podiatr Med Surg 35:323–330. https://doi.org/10.1016/j.cpm.2018.02.007
Kunze KN, Burnett RA, Wright-Chisem J et al (2020) Adipose-derived mesenchymal stem cell treatments and available formulations. Curr Rev Musculoskelet Med 13:264–280. https://doi.org/10.1007/s12178-020-09624-0
Bianchi F, Maioli M, Leonardi E et al (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22:2063–2077. https://doi.org/10.3727/096368912X657855
Tremolada C, Colombo V, Ventura C (2016) Adipose tissue and mesenchymal stem cells: state of the art and lipogems® technology development. Current Stem Cell Reports 2:304–312. https://doi.org/10.1007/s40778-016-0053-5
Im G-I, Shin Y-W, Lee K-B (2005) Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarth Cartilage 13:845–853. https://doi.org/10.1016/j.joca.2005.05.005
Peng L, Jia Z, Yin X et al (2008) Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Develop 17:761–773. https://doi.org/10.1089/scd.2007.0217
Orbay H, Tobita M, Mizuno H (2012) Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int 2012:461718. https://doi.org/10.1155/2012/461718
Alvarez-Viejo M, Menendez-Menendez Y, Blanco-Gelaz MA et al (2013) Quantifying mesenchymal stem cells in the mononuclear cell fraction of bone marrow samples obtained for cell therapy. Transpl Proc 45:434–439. https://doi.org/10.1016/j.transproceed.2012.05.091
Baer PC, Geiger H (2012) Adipose-derived mesenchymal stromal/stem cells: tissue localization, characterization, and heterogeneity. Stem Cells Int 2012:812693. https://doi.org/10.1155/2012/812693
Bunnell BA, Flaat M, Gagliardi C et al (2008) Adipose-derived stem cells: isolation, expansion and differentiation. Methods (San Diego, Calif) 45:115–120. https://doi.org/10.1016/j.ymeth.2008.03.006
Aronowitz JA, Lockhart RA, Hakakian CS (2015) Mechanical versus enzymatic isolation of stromal vascular fraction cells from adipose tissue. Springerplus 4:713. https://doi.org/10.1186/s40064-015-1509-2
Mashiko T, Wu S-H, Feng J et al (2017) Mechanical micronization of lipoaspirates: squeeze and emulsification techniques. Plast Reconstr Surg 139:79–90. https://doi.org/10.1097/PRS.0000000000002920
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. https://doi.org/10.1136/BMJ.N71
Slim K, Nini E, Forestier D et al (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73:712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Freitag J, Wickham J, Shah K, Tenen A (2020) Effect of autologous adipose-derived mesenchymal stem cell therapy in the treatment of an osteochondral lesion of the ankle. BMJ Case Reports 13:e234595. https://doi.org/10.1136/bcr-2020-234595
Kim YS, Lee M, Koh YG (2016) Additional mesenchymal stem cell injection improves the outcomes of marrow stimulation combined with supramalleolar osteotomy in varus ankle osteoarthritis: short-term clinical results with second-look arthroscopic evaluation. J Exper Orthopaed 3:12. https://doi.org/10.1186/s40634-016-0048-2
Kim YS, Koh YG (2016) Injection of mesenchymal stem cells as a supplementary strategy of marrow stimulation improves cartilage regeneration after lateral sliding calcaneal osteotomy for varus ankle osteoarthritis: clinical and second-look arthroscopic results. Arthrosc: J Arthrosc Relat Surg Off Publ Arthrosc Associat North America Int Arthrosc Assoc 32:878–889. https://doi.org/10.1016/j.arthro.2016.01.020
Kim YS, Lee HJ, Choi YJ et al (2014) Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med 42:2424–2434. https://doi.org/10.1177/0363546514541778
Kim YS, Park EH, Kim YC, Koh YG (2013) Clinical outcomes of mesenchymal stem cell injection with arthroscopic treatment in older patients with osteochondral lesions of the talus. Am J Sports Med 41:1090–1099. https://doi.org/10.1177/0363546513479018
Shimozono Y, Dankert JF, Kennedy JG (2021) Arthroscopic debridement and autologous micronized adipose tissue injection in the treatment of advanced-stage posttraumatic osteoarthritis of the ankle. Cartilage 13:1337S-1343S. https://doi.org/10.1177/1947603520946364
D’ambrosi R, Indino C, Maccario C et al (2018) Autologous microfractured and purified adipose tissue for arthroscopic management of osteochondral lesions of the talus. J Visual Exper: JoVE 2018:56395. https://doi.org/10.3791/56395
Natali S, Screpis D, Farinelli L et al (2021) The use of intra-articular injection of autologous micro-fragmented adipose tissue as pain treatment for ankle osteoarthritis: a prospective not randomized clinical study. Int Orthop 45:2239–2244. https://doi.org/10.1007/s00264-021-05093-3
Yin F, Cai J, Zen W et al (2015) Cartilage regeneration of adipose-derived stem cells in the TGF-β1-immobilized PLGA-Gelatin scaffold. Stem Cell Rev Reports 11:453–459. https://doi.org/10.1007/s12015-014-9561-9
Sukarto A, Yu C, Flynn LE, Amsden BG (2012) Co-delivery of adipose-derived stem cells and growth factor-loaded microspheres in RGD-grafted N-methacrylate glycol chitosan gels for focal chondral repair. Biomacromol 13:2490–2502. https://doi.org/10.1021/bm300733n
Zhu M, Cohen SR, Hicok KC et al (2013) Comparison of three different fat graft preparation methods: gravity separation, centrifugation, and simultaneous washing with filtration in a closed system. Plast Reconstr Surg 131:873–880. https://doi.org/10.1097/PRS.0b013e31828276e9
Dragoo JL, Carlson G, McCormick F et al (2007) Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng 13:1615–1621. https://doi.org/10.1089/ten.2006.0249
Pers Y-M, Rackwitz L, Ferreira R et al (2016) Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med 5:847–856. https://doi.org/10.5966/sctm.2015-0245
Jo CH, Chai JW, Jeong EC et al (2017) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a 2-year follow-up study. Am J Sports Med 45:2774–2783. https://doi.org/10.1177/0363546517716641
Frigg A, Nigg B, Hinz L et al (2010) Clinical relevance of hindfoot alignment view in total ankle replacement. Foot Ankle Int 31:871–879. https://doi.org/10.3113/FAI.2010.0871
Chopra V, Stone P, Ng A (2017) Supramalleolar osteotomies. Clin Podiatr Med Surg 34:445–460. https://doi.org/10.1016/j.cpm.2017.05.003
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, drafting, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arceri, A., Mazzotti, A., Artioli, E. et al. Adipose-derived stem cells applied to ankle pathologies: a systematic review. Musculoskelet Surg 108, 1–9 (2024). https://doi.org/10.1007/s12306-023-00798-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-023-00798-7