Abstract
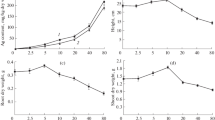
Due to the increased production and release of silver nanoparticles (AgNPs) in the environment, the concerns about the possibility of toxicity and oxidative damage to plant ecosystems should be considered. In the present study, the effects of different concentrations of AgNPs (0, 0.5, 1, 2, 3 and 4 g/L) synthesized using the extract of camelina (Camelina sativa) leaves on the growth and the biochemical traits of camelina seedlings were investigated. The results showed that AgNPs significantly increased Ag accumulation in the roots and shoots which decreased the growth and photosynthetic pigments of camelina seedlings. The highest decrease in the height and total dry weight was observed by 53.1 and 61.8% under 4 g/L AgNPs, respectively over control plants. AgNPs application over 2 g/L enhanced the accumulation of proline, malondialdehyde, hydrogen peroxide and methylglyoxal, and up-regulated the activity of antioxidant enzymes (superoxide dismutase, catalase, ascorbate peroxidase and glutathione reductase) and glyoxalase (glyoxalase I and II) system which indicates oxidative stress induction in camelina seedlings. Moreover, AgNPs reduced ASA and GSH contents and increased DHA and GSSG contents, hence disrupting the redox balance. These results showed that AgNPs at 4 g/L had the most toxic effects on the camelina growth. Therefore, increasing oxidative stress markers and the activity of antioxidant enzymes and enzymes involved in glyoxalase system indicated the oxidative stress induced by AgNPs treatments over 2 g/L as well as the induction of antioxidant defense systems to combat AgNPs-induced oxidative stress.






Similar content being viewed by others
References
Anna B, Barbara K, Magdalena O (2018) How the surface properties affect the nanocytotoxicity of silver? study of the influence of three types of nanosilver on two wheat varieties. Acta Physiol Plant 40:31
Bagherzadeh Homaee M, Ehsanpour AA (2016) Silver nanoparticles and silver ions: oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic Environ Biotechnol 57:544–553
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P (2010) The release of nanosilver from consumer products used in the home. J Environ Qual 39:1875–1882
Berti M (2016) Camelina uses, genetics, genomics, production, and management. Ind Crops Prod 94:690–710
Bondarenko O, Juganson K, Ivask A, Kasemets K, Mortimer M, Kahru A (2013) Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Arch Toxicol 87:1181–1200
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42:4583–4588
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245(1):85–96
Fabrega J, Luoma SN, Tyler CR, Galloway TS, Lead JR (2011) Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ Intl 37:517–531
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133(1):21–25
Gerami M, Ghorbani A, Karimi S (2018) Role of salicylic acid pretreatment in alleviating cadmium-induced toxicity in Salvia officinalis L. Iranian J Plant Biol 10(1):81–95
Ghorbani A, Ghasemi Omran VO, Razavi SM, Pirdashti H, Ranjbar M (2019) Piriformospora indica confers salinity tolerance on tomato (Lycopersicon esculentum Mill.) through amelioration of nutrient accumulation, K+/Na+ homeostasis and water status. Plant Cell Rep 38:1151–1163
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018a) Piriformospora indica inoculation alleviates the adverse effect of NaCl stress on growth, gas exchange and chlorophyll fluorescence in tomato (Solanum lycopersicum L.). Plant Biol 20:729–736
Ghorbani A, Razavi SM, Ghasemi Omran VO, Pirdashti H (2018b) Piriformospora indica alleviates salinity by boosting redox poise and antioxidative potential of tomato. Russ J Plant Physiol 65:898–907
Ghorbani A, Tafteh M, Roudbari N, Pishkar L, Zhang W, Wu C (2020) Piriformospora indica augments arsenic tolerance in rice (Oryza sativa) by immobilizing arsenic in roots and improving iron translocation to shoots. Ecotoxicol Environ Saf 209:111793
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143(3):1758–1776
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys, 125(1):189–198
Jiang HS, Li M, Chang FY, Li W, Yin LY (2012) Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem 31(8):1880–1886
Jiang HS, Qiu XN, Li GB, Li W, Yin LY (2014) Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ Toxicol Chem 33(6):1398–1405
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants. CRC Press, Washington, DC
Kanounboule M, Vicente J, Nabais C, Prasad M, Freitas H (2009) Ecophysiological tolerance of duckweeds exposed to copper. Aquat Toxicol 91:1–9
Khan I, Raza MA, Khalid M, Awan SA, Raja NI, Zhang X, Min S, Wu BC, Hassan MJ, Huang L (2019) Physiological and biochemical responses of pearl millet (Pennisetum glaucum L.) seedlings exposed to silver nitrate (AgNO3) and silver nanoparticles (AgNPs). Int J Environ Res Public Health 16(13):2261.
Kishor PK, Sangam S, Amrutha R, Laxmi PS, Naidu K, Rao K, Rao S, Reddy K, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382
Ma C, Chhikara S, Xing B, Musante C, White JC, Dhankher OP (2013) Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustain Chem Eng 1:768–778
Mahmood Q, Ahmad R, Kwak SS, Rashid A, Anjum NA (2010) Ascorbate and glutathione: protectors of plants in oxidative stress. In: Anjum NA, Chan MT, Umar S (eds) Ascorbate-glutathione pathway and stress tolerance in plants. Springer, Dordrecht, The Netherlands, pp 209–229
Mallick N (2004) Copper-induced oxidative stress in the chlorophycean microalga Chlorella vulgaris: response of the antioxidant system. J Plant Physiol 161:591–597
Massarsky A, Dupuis L, Taylor J, Eisa-Beygi S, Strek L, Trudeau VL, Moon TW (2013) Assessment of nanosilver toxicity during zebrafish (Danio rerio) development. Chemosphere 92:59–66
Mostofa MG, Seraj ZI, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251(6):1373–1386.
Nair PM, Chung IM (2014) Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 112:105–113
Najafi S, Razavi SM, Khoshkam M, Asadi A (2020) Effects of green synthesis of sulfur nanoparticles from Cinnamomum zeylanicum barks on physiological and biochemical factors of Lettuce (Lactuca sativa). Physiol Mol Biol Plants 26(5):1055–1066
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nath S, Panda P, Mishra S, Dey M, Choudhury S, Sahoo L, Panda SK (2014) Arsenic stress in rice: redox consequences and regulation by iron. Plant Physiol Biochem 80:203–210
Newton KM, Puppala HL, Kitchens CL, Colvin VL, Klaine SJ (2013) Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environ Toxicol Chem 32:2356–2364
Oukarroum A, Bras S, Perreault F, Popovic R (2012) Inhibitory effects of silver nanoparticles in two green algae, Chlorella vulgaris and Dunaliella tertiolecta. Ecotox Environ Safe 78:80–85
Pilgeram AL (2007) Camelina sativa, a montana omega-3 and fuel crop. In: Janick J, Whipkey A (eds) Issues in new crops and new uses. ASHS Press, Alexandria, pp 129–131
Principato GB, Rosi G, Talesa V, Govannini E, Uolila L (1987) Purification and characterization of two forms of glyoxalase II from the liver and brain of wistar rats. Biochim Biophys Acta Protein Struct Molec Enzym 911(3):349–355
Raghunandan D, Bedre MD, Basavaraja S, Sawle B, Manjunath S, Venkataraman A (2010) Rapid synthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf B Biointerfaces 79:235–240
Rahman A, Mostofa MG, Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2015) Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res Int 2015
Saxena M, Deb Roy S, Singla-Pareek SL, Sopory SK, Bhalla-Sarin N (2011) Overexpression of the glyoxalase II gene leads to enhanced salinity tolerance in Brassica juncea. Open Plant Sci J 5:23–28
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2008) Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res 17(2):171–180
Sudhasree S, Shakila Banu A, Brindha P, Kurian GA (2014) Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol Environ Chem 96:743–754
Sunita RB, Veera RG, Tushar KG, Robert VT, Sudarshan KL (2011) Gold, silver, and palladium nanoparticle/nano-agglomerate generation, collection, and characterization. J Nanopart Res 13:6591–6601
Thakkar KN, Mhatre SS, Parikh RY (2010) Biological synthesis of metallic nanoparticles. Nanomedicine 6:257–262
Torres SK, Campos VL, Leon CG, Rodr´ıguez-Llamazares SM, Rojas SM, Gonzalez M, Smith C, Mondaca MA (2012) Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity. J Nanopart Res 14:1236
Tripathi DK, Singh S, Singh S, Srivastava PK, Singh VP, Singh S, Prasad SM, Singh PK, Dubey NK, Pandey AC, Chauhan DK (2017) Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem 110:167–177
Vishwakarma K, Shweta UN, Singh J, Liu S, Singh VP, Prasad SM, Chauhan DK, Tripathi DK, Sharma S (2017) Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front Plant Sci 8:1501
Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van De Meent D (2009) Nano-silver-a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 3:109–138
Wild R, Ooi L, Srikanth V, Mu¨nch G, (2012) A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal Bioanal Chem 403(9):2577–2581
Xing W, Huang WM, Liu GH (2010) Effect of excess iron and copper on physiology of aquatic plant Spirodela polyrrhiza (L.) Schleid. Environ Toxicol 25:103–112
Xiu ZM, Ma J, Alvarez PJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environ Sci Technol 45:9003–9008
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12:4271–4275
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11(1):1–11
Yang Y, Wang J, Xiu Z, Alvarez PJ (2013) Impacts of silver nanoparticles on cellular and transcriptional activity of nitrogen-cycling bacteria. Environ Toxicol Chem 32:1488–1494
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367
Yin N, Liu Q, Liu J, He B, Cui L, Li Z, Yun Z, Qu G, Liu S, Zhou Q, Jiang G (2013) Silver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stress. Small 9:1831–1841
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30(9):955–963
Zare Z, Pishkar L, Iranbakhsh A, Talei D (2020) Physiological and molecular effects of silver nanoparticles exposure on purslane (Portulaca oleracea L.). Russ J Plant Physiol 67:521–528
Zhang Y, Ferguson SA, Watanabe F, Jones Y, Xu Y, Biris AS, Hussain S, Ali SF (2013) Silver nanoparticles decrease body weight and locomotor activity in adult male rats. Small 9:1715–1720
Zhang HY, Jiang YN, He ZY, Ma M (2005) Cadmium accumulation and oxidative burst in garlic (Allium sativum). J Plant Physiol 162:977
Zhao CM, Wang WX (2011) Comparison of acute and chronic toxicity of silver nanoparticles and silver nitrate to Daphnia magna. Environ Toxicol Chem 30:885–892
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mirmoeini, T., Pishkar, L., Kahrizi, D. et al. Phytotoxicity of green synthesized silver nanoparticles on Camelina sativa L. Physiol Mol Biol Plants 27, 417–427 (2021). https://doi.org/10.1007/s12298-021-00946-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-021-00946-y