Abstract
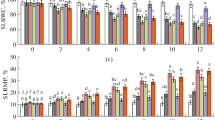
Polyamines (PAs) are positively charged molecules known to mitigate drought stress; however, little is known about their mechanism of alleviating drought stress. We investigated the effects of PAs exogenously applied as a seed primer and as a foliar spray on the growth, membrane stability (MS), electrolyte leakage (EL), Na+ and K+ cations, reactive oxygen species (ROS), catalase (CAT; EC 1.11.1.6) and guaiacol peroxidase (GPX; EC 1.11.1.7) activity and chloroplast ultra-structure in wheat (Triticum aestivum L.; cv. Sakha-94) under drought stress. Three PA solutions, namely, putrescine, spermine and a mixture of the two (Mix), were each applied at a concentration of 100 µM. Our study demonstrated that the retardation of chlorophyll loss and elevation of Rubisco levels were involved in PA-enhanced growth under drought stress. These relationships were mainly reflected in elevated fresh weight and dry weight in response to foliar spraying with all PA solutions and seed priming with the Mix solution. The elevated growth seemed to be due to increased photosynthetic pigments, protein and Rubisco. In contrast, drought decreased growth, photosynthetic pigments, protein and Rubisco. MS was enhanced by PAs applied as a seed primer or foliar spray, as shown by clear reductions in EL %, malondialdehyde (MDA) content and the Na+/K+ ratio as well as reduced ROS markers and elevated CAT (but not GPX) activity. Further study showed that the Mix solution of PAs, applied either during seed priming or as a foliar spray, improved chloroplast ultra-structure, suggesting that improvements in Rubisco and photosynthetic pigments were involved in PA maintenance of chloroplast stability. Therefore, the present study showed that elevated CAT activity is the main mechanism through which PAs reduce ROS and MDA, thereby improving MS and protecting mesophyll cells structurally and functionally under drought stress in wheat.






Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- CHL a :
-
Chlorophyll a
- CHL b :
-
Chlorophyll b
- DAB:
-
3′,3′-Diaminobenzidine
- DAS:
-
Days after sowing
- DW:
-
Dry weight
- Ec:
-
Electrical conductivity
- FW:
-
Fresh weight
- GPX:
-
Guaiacol peroxidase
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- MS:
-
Membrane stability
- NBT:
-
Nitro blue tetrazolium chloride
- PAs:
-
Polyamines
- POD:
-
Peroxidase
- Put:
-
Putrescine
- ROS:
-
Reactive oxygen species
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TEM:
-
Transmission electron microscopy
References
Alcázar R, Altabella T, Marco F et al (2010) Polyamines : molecules with regulatory functions in plant abiotic stress tolerance. Planta 231:1237–1249. https://doi.org/10.1007/s00425-010-1130-0
Ali Q, Ashraf M, Anwar F (2009) Physico-chemical attributes of seed oil from drought stressed sunflower (Helianthus annuus L.) plants. Grasas Aceites 60:475–481. https://doi.org/10.3989/gya.021009
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asada K, Takahashi M (1987) Production and scavenging of active oxygen in photosynthesis. In: Kyle DJ, Osborne CB, Arntzen CJ (eds) Photoinhibition. Elsevier, Amsterdam, pp 227–287
Basu S, Ramegowda V, Kumar A, Pereira A (2016) Plant adaptation to drought stress. F1000Research 5:1554
Botella-Pavía P, Rodríguez-Concepción M (2006) Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant 126:369–381
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chance B, Maehly SK (1955) Assay of catalase and peroxidases. Methods Enzymol 2:764–775
Chen F, Wang F, Wu F et al (2010) Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681
Cvikrová M, Gemperlová L, Martincová O, Vanková R (2013) Plant Physiology and Biochemistry Effect of drought and combined drought and heat stress on polyamine metabolism in proline-over-producing tobacco plants. Plant Physiol Biochem 73:7–15. https://doi.org/10.1016/j.plaphy.2013.08.005
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32:79–91
Dondini L, Del Duca S, Dall’Agata L et al (2003) Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates. Planta 217:84–95
Ebeed, HT (2019) Omics Approaches for Developing Abiotic Stress Tolerance in Wheat. In: Wheat Production in Changing Environments. Springer, Singapore, pp 443–463
Ebeed HT, Hassan NM, Aljarani AM (2017) Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol Biochem 118:438–448. https://doi.org/10.1016/j.plaphy.2017.07.014
Ebeed HT, Stevenson SR, Cuming AC, Baker A (2018) Conserved and differential transcriptional responses of peroxisome associated pathways to drought, dehydration and ABA. J Exp Bot 69:4971–4985. https://doi.org/10.1093/jxb/ery266
Ebeed HT, Hassan NM, Keshta MM, Hassanin OS (2019) Comparative analysis of seed yield and biochemical attributes in different sunflower genotypes under different levels of irrigation and salinity. Egypt J Bot 59:339–355. https://doi.org/10.21608/ejbo.2019.5043.1205
Farooq M, Wahid A, Kobayashi N et al (2009a) Plant drought stress : effects, mechanisms and management. Agron Sustain Dev 29:185–212. https://doi.org/10.1051/agro:2008021
Farooq M, Wahid ÆA, Lee D (2009b) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945. https://doi.org/10.1007/s11738-009-0307-2
Feller U, Anders I, Mae T (2007) Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. J Exp Bot 59:1615–1624
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ 28:1056–1071
Ghoulam C, Foursy A, Fares K (2002) Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot 47:39–50
Grigorova B, Vassileva V, Klimchuk D et al (2012) Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J Plant Interact 7:204–213. https://doi.org/10.1080/17429145.2011.654134
Gupta S, Agarwal VP, Gupta NK (2012) Efficacy of putrescine and benzyladenine on photosynthesis and productivity in relation to drought tolerance in wheat (Triticum aestivum L.). Physiol Mol Biol Plants. https://doi.org/10.1007/s12298-012-0123-9
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Nahar K, Rahman A et al (2018) Exogenous nitric oxide donor and arginine provide protection against short-term drought stress in wheat seedlings. Physiol Mol Biol Plants 24:993–1004
Hassan NM, El-Sayed AKA, Ebeid HT, Nemat Alla MM (2011) Molecular aspects in elevation of sunflower tolerance to drought by boron and calcium foliar sprays. Acta Physiol Plant. https://doi.org/10.1007/s11738-010-0585-8
Hassan N, El-bastawisy Z, Ebeed H, Nemat Alla M (2015a) Role of defense enzymes, proteins, solutes and Δ1-pyrroline-5-carboxylate synthase in wheat tolerance to drought. Rend Lincei. https://doi.org/10.1007/s12210-015-0429-y
Hassan NM, El-Bastawisy ZM, El-Sayed AK et al (2015b) Roles of dehydrin genes in wheat tolerance to drought stress. J Adv Res. https://doi.org/10.1016/j.jare.2013.11.004
He L, Nada K, Kasukabe Y, Tachibana S (2002) Enhanced susceptibility of photosynthesis to low-temperature photoinhibition due to interruption of chill-induced increase of S-adenosylmethionine decarboxylase activity in leaves of spinach (Spinacia oleracea L.). Plant Cell Physiol 43:196–206
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoffmann WA, Poorter H (2002) Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42
Hunt J (1982) Dilute hydrochloric acid extraction of plant material for routine cation analysis. Commun Soil Sci Plant Anal 13:49–55
Kaur G, Asthir B (2017) Molecular responses to drought stress in plants. Biol Plant 61:201–209
Kirchhoff H, Haase W, Haferkamp S et al (2007) Structural and functional self-organization of Photosystem II in grana thylakoids. Biochim Biophys Acta (BBA)-Bioenergetics 1767:1180–1188
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680
Liu Y, Liang H, Lv X et al (2016a) Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem 100:113–129
Liu Y, Xu H, Wen X, Liao Y (2016b) Effect of polyamine on seed germination of wheat under drought stress is related to changes in hormones and carbohydrates. J Integr Agric 15:2759–2774
Metzner H, Rau H, Senger H (1965) Untersuchungen zur Synchronisierbarkeit einzelner Pigmentmangel-Mutanten von Chlorella (Studies on synchronization of some pigment-deficient Chlorella mutants). Planta 65:186–194
Mihailović N, Lazarević M, Dželetović Z et al (1997) Chlorophyllase activity in wheat, Triticum aestivum L. leaves during drought and its dependence on the nitrogen ion form applied. Plant Sci 129:141–146
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nahar K, Hasanuzzaman M, Rahman A et al (2016) Polyamines confer salt tolerance in Mung bean (Vigna radiata L.) by reducing sodium uptake, improving nutrient homeostasis, antioxidant defense, and methylglyoxal detoxification systems. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01104
Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Nielsen DC, Halvorson AD, Vigil MF (2010) Critical precipitation period for dryland maize production. Field Crops Res 118:259–263
Osman HS (2015) Enhancing antioxidant–yield relationship of pea plant under drought at different growth stages by exogenously applied glycine betaine and proline. Ann Agric Sci 60:389–402
Shabala S, Cuin TA, Pottosin I (2007) Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett 581:1993–1999. https://doi.org/10.1016/j.febslet.2007.04.032
Silveira V, de Vita AM, Macedo AF et al (2013) Morphological and polyamine content changes in embryogenic and non-embryogenic callus of sugarcane. Plant Cell Tissue Organ Cult 114:351–364
Spreitzer RJ (1993) Genetic dissection of Rubisco structure and function. Annu Rev Plant Biol 44:411–434
Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11:1187–1194
Yu C-W, Murphy TM, Lin C-H (2003) Hydrogen peroxide-induced chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Zheleva D, Tsonev T, Sergiev I, Karanov E (1994) Protective effect of exogenous polyamines against atrazine in pea plants. J Plant Growth Regul 13:203
Zivcak M, Brestic M, Balatova Z et al (2013) Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth Res 117:529–546
Acknowledgements
We are grateful to Prof. Zakaria A. Baka for providing help with the ultra-structure analysis. This work was performed at Damietta University, Egypt. Scholarship from Sabha University (A.A), Libya (No. 393), supported this research.
Author information
Authors and Affiliations
Contributions
NMH supervised the experiments and critically revised the manuscript; HTE conceived and designed the research, supervised the experiments, performed the TEM photography, analysed the data statistically, interpreted the results and wrote the manuscript; and AMA performed the plant growth experiments and analysed the physiological parameters.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hassan, N., Ebeed, H. & Aljaarany, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol Mol Biol Plants 26, 233–245 (2020). https://doi.org/10.1007/s12298-019-00744-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00744-7