Abstract
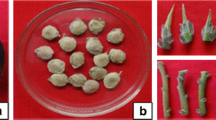
Efficient methods were developed for both in vitro seed germination and micropropagation of an economically important dye yielding multipurpose tree, Bixa orellana L. Mature seeds were inoculated onto Murashige and Skoog (MS) medium supplemented with different concentrations of gibberellic acid (GA3). Highest frequency of germination (93.3 %) was recorded on medium supplemented with 3 μM GA3 against 13.33 % in control. Nodal explants cultured on MS medium fortified with 5 μM isopentanyl adenine (2-iP) produced maximum explants response (93.3 %) and highest number of shoots (35.71). Addition of relatively higher concentration (15 μM) of benzyl adenine (BA) resulted in the production of significantly (P < 0.05) reduced number of shoots (12.66). Sucrose at 87.6 mM was found to be the best carbohydrate source for multiple shoot induction compared to glucose and fructose. Regenerated shoots (3–4 cm) were rooted (95.5 %) on agar gelled MS medium supplemented with 10 μM indole-3-butyric acid (IBA). In vitro developed plantlets with well-developed roots were potted and acclimatized initially in the growth chamber and then moved to a green house with 83.3 % survival. The present protocol avoids the use of auxins in shoot multiplication medium, which will lower the cost, avoid callus formation and thus reduces the possibility of somaclonal variation in the regenerated plants. The method is efficient to produce over 32,000 hardened plants within a 10-month culture period starting from a single nodal explant.

Similar content being viewed by others
References
Almeida JL, Almeida FCG, Nunes RDEP, Almeida FAG (1996) Bud initiation in leaf explants of annatto seedlings in different cytokinins. Cinencia Rural 26:45–49
Aparnathi K, Lata R, Sharma R (2003) Annatto (Bixa orellana L.): its cultivation, preparation and usage. Int J Trop Agric 8:80–88
Benson E, Danahar JE, Pimbley IM, Anderson CT, Wake JE, Daley S, Adams LK (2000) In vitro propagation of Primula scotica: a rare Scottish plant. Biodivers Conserv 9:711–726
Carvalho PR (1999) Technological advances and perspectives. Arch Latinoam Nutr 49:71–73
Collins P, Hughes S (1991) Report in prospective in natural food symposium. Oversea food Ltd., England
D’Souza MC, Sharon M (2001) In vitro clonal propagation of annatto (Bixa orellana L.). In Vitro Cell Dev Biol-Plant 37:168–172
De Klerk G, Brugge J, Marinova S (1997) Effectiveness of indole-acetic acid, indole butyric acid and naphthalene acetic acid during adventitious root formation in vitro in Malus Jork 9. Plant Cell Tiss Organ Cult 49:39–44
Debnath SC, McRae KB (2001) An efficient in vitro shoot propagation of cranberry (Vaccinium macrocarpon AIT.) by axillary bud proliferation. In Vitro Cell Dev Biol-Plant 37:243–249
George EF (1996) Plant propagation by tissue culture, Part 2: in practice. Exegetics Ltd, London, p 799
Hartman HT, Kester KE (1983) Plant propagation and practices. Prentice Hall, India Pvt. Ltd, New Delhi
Husain MK, Anis M, Shahzad A (2007) In vitro propagation of Indian kino (Pterocarpus marsupim Roxb.) using Thidiazuron. In Vitro Cell Dev Biol-Plant 43:59–64
Joseph N, Siril EA, Nair GM (2010) Imbibition duration, seed treatment, seed mass and population influence germination of annatto (Bixa orellana L.) seeds. Seed Technol 32:37–45
Khan PSSV, Prakash E, Rao KR (2002) Callus induction and plantlet regeneration in Bixa orellana L., an annatto-yielding tree. In Vitro Cell Dev Biol-Plant 38:186–190
Larkin PJ, Scowcroft WR (1981) Somaclonal variation- a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Ludwig-Muller J (2000) Indole-3-butyric acid in plant growth and development. Plant Growth Regul 32:219–230
Marcotrigiano M, McGlew SP (1991) A two-stage micropropagation system for cranberries. J Am Soc Hort Sci 116:911–916
Marks TM, Simpson SE (2000) Interaction of explants type and indole-3-butyric acid during rooting in vitro in a range of difficult and easy-to-root woody plants. Plant Cell Tiss Organ Cult 62:65–74
Mercier H, Kerbauy GB (1997) Micropropagation of ornamental bromeliads (Bromeliaceae). Biotechnol Agric For 40:43–57
Nassar AH, Khalifa SF, Hammouda FM, Shams KA (2001) In vitro propagation of Bixa orellana L. (annatto) an economically important plant newly introduced to Egypt. Egyptian J Bot 41:241–253
Neto VBP, de Botelho MN, Aguiar R, Silva EAM, Otoni WC (2003) Somatic embryogenesis from immature zygotic embryos of annatto (Bixa orellana L.). In Vitro Cell Dev Biol-Plant 39:629–634
Neto VPB, Reis LB, Finger FL, Barros RS, Carvalho CR, Otoni WC (2009) Involvement of ethylene in the rooting of seedling shoot cultures of Bixa orellana L. In Vitro Cell Dev Biol-Plant 45:693–700
Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA (2007) Organogenesis from cotyledon and hypocotyls derived explants of japhara (Bixa orellana L.). Act Bot Croat 66:153–160
Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA (2008) Mass multiplication of Bixa orellana L. through tissue culture for commercial propagation. Ind Crops and Prod 28:122–127
Parimalan R, Giridhar P, Gururaj HB, Ravishankar GA (2009) Micropropagation of Bixa orellana using phytohormones and triacontanol. Biol Plant 53:347–350
Parimalan R, Giridhar P, Ravishankar GA (2010) Enhanced shoot organogenesis in Bixa orellana L. in the presence of putrescine and silver nitrate. Plant Cell Tiss Organ Cult 105:285–290
Ramamurthy N, Savithramma N, Usha R, Swamy PM (1999) Multiple shoot induction and regeneration of japhara (Bixa orellana L.) through axillary bud derived callus cultures. J Plant Biol 26:231–235
Satyanarayana A, Rao PG, Rao DG (2003) Chemistry, processing and toxicology of annatto (Bixa orellana L.). J Food Sci Technol 40:131–141
Sharon M, D’Souza MC (2000) In vitro clonal propagation of annatto (Bixa orellana L.). Curr Sci 78:1532–1535
Siril EA, Dhar U (1997) Micropropagation of mature Chinese tallow tree (Sapium sebiferum Roxb.). Plant Cell Rep 16:637–640
Snedecor GW, Cochran WG (1962) Statistical methods, 4th edn. The Iowa State University Press, Iowa
Tiwari SK, Tiwari KP, Siril EA (2002) An improved micropropagation protocol for teak. Plant Cell Tiss Organ Cult 71:1–6
Acknowledgements
We thank Dr. Ashalatha S Nair, Professor and Head, Department of Botany for providing the facilities and Kerala State Council for Science, Technology and Environment (KSCSTE) Govt. of Kerala, Thiruvananthapuram, India, for the financial support (No.028/SRSLS/2007/CSTE).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joseph, N., Siril, E.A. & Nair, G.M. An efficient in vitro propagation methodology for Annatto (Bixa orellana L.). Physiol Mol Biol Plants 17, 263–270 (2011). https://doi.org/10.1007/s12298-011-0073-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-011-0073-7