Abstract
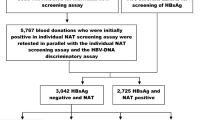
Nucleic acid Amplification testing (NAT) has helped improve blood safety and detect window period and Occult Hepatitis B infections (OBI) This study was aimed at determining the following in blood donors: 1. seroprevalence of HIV, HBV & HCV, malarial parasite and Syphlis 2. NAT and seroyield for HIV, HBV and HCV 3. viral load in NAT yield donations 4. Pattern of HBV serological markers in HBV NAT yield donations 80,809 blood donations were screened over an 8 year period (2012–2019) for antiHIV I and II, HBsAg, antiHCV antibodies, malarial parasite and VDRL. Seronegative samples were tested by NAT using a multiplex PCR in a pool of six. NAT yield samples were tested for viral load and HBV serological markers. Seropositive samples were tested for NAT and checked for seroyield. SPSS windows version 24.0 was used for statistical analysis. 1.07% of blood donors were found to be seropositive with 0.08%, 0.86%, 0.09%, 0.03% and 0 for anti HIV I and II, HBsAg, antiHCV, VDRL and Malarial parasite respectively. Out of 79,938 seronegative samples, 20 samples (0.025%) were NAT positive for Hepatitis B with a NAT yield OF 1:3997. Out of the 20 NAT positive samples, 17 were OBI and three were window period infections. 14 NAT yield samples subjected to a HBV viral load assay showed a range of < 6–146 IU/ml. Minipool NAT in pools of six is able to indentify both OBI and window period infections. NAT could significantly improve the blood safety in a resource limited setting like India.
Similar content being viewed by others
Data Availability
Available on request.
References
Screening donated blood for Transfusion Transmissible Infections Recommendations WHO (2009) Available at :https://www.who.int/bloodsafety/ScreeningTTI.pdf. Accessed from 20 Nov 2020
Hepatitis B Fact sheets WHO 27.07.2020. https://www.who.int/newsroom/fact-sheets/detail/hepatitis-BAccessed Available from 20 Nov 2020
Hepatitis C Fact Sheets WHO 27.07.2020. Available at :https://www.who.int/news-room/fact-sheets/detail/hepatitis-C. Accessed 20 Nov .2020
HIV/AIDS Fact sheets WHO 06.07.2020. Available at :https://www.who.int/news-room/fact-sheets/detail/ HIV/AIDS. Accessed from 20 Nov 2020
Bolton-Maggs PHB, Poles D On behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2016 annual SHOT report. SHOT Office, Manchester, UK. Available at: https://www.who.int/news-room/fact-sheets/detail/HIV/AIDS. Accessed from 20 Nov 2020
Ainley LI, Hewitt PE (2018) Haematology patients and the risk of transfusion transmitted infection. Br J Haematol 180:473–483
De Paula EV, Goncales FL (2005) Transfusion Transmitted infections among multitransufsed patients in Brazil. J Clin Vorol 34(Suppl2):S27–S32
Ray G (2017) Current scenario of Hepatitis B and its treatment in India. J Clin Transl Hepatol 5(3):277–296
Vidja PJ, Vachhani JH, Sheikh SS, Santwani PM (2011) Blood transfusion transmitted infections in multiple blood transfused patients of beta Thalassemia. Indian J Haematol Blood Transfus 27(2):65–69
Yuen MF, Wong DKH, Lee CK et al (2011) Transmissibility of Hepatitis B Virus ( HBV) infection through blood transfusion from blood donors with occult HBV infection. Clin Infect Dis 52(5):624–632
Candotti D, Allain JP (2009) Transfusion transmitted hepatitis B virus infection. J Hepatol 51(4):798–809
Allain JP (2004) Occult Hepatitis B virus infection: implication in transfusion. Vox Sang 86(2):83–91
Su TH, Chen PJ, Chen TC et al (2011) The clinical significance of occult Hepatitis b transfusion in Taiwan- a look back study. Transfus Med 21(1):33–41
Vermeulen M, Lelie N, Sykes W et al (2009) Impact of individual donation Nucleic acid testing on risk of Human Immunodeficiency Virus, Hepatitis B Virus and Hepatitis C Virus transmission by Blood Trasnfusion in South Afrcia. Transfusion 49(6):1115–1125
Gazette of India Part-II Section 3 Subsection (i) March 2020 : 1–30
Ghosh K, Mishra K (2017) Nucleic acid amplification testing in Indian Blood Banks: a review with perspectives. Indian J Pathol Microbiol 60:313–318
Esposito A, Sabia C, Iannone C et al (2017) Occult hepatitis infection in transfusion medicine: screening policy and assessment of current use of anti- HBc testing. Transfus Med Haemother 44:263–272
Satake M, Taira R, Yugi H et al (2007) Infectivity of blood components with low Hepatitis B virus DNA levels identified in a lookback program. Transfusion 47:1197–1205
Datta S, Khillan K, Ranjan V, Wattal C (2019) Nucleic acid amplification test: Bridging the gap in blood safety and re-evaluationof blood screening for cryptic transfusion transmitted among Indian donors. Indian J Med Res 149:389–395
Rawat A, Diwaker P, Gogoi P, Singh B (2017) Seroprevalence and changing trends of transfusion transmitted infections amongst blood donors in a Regional Blood Transfusion Centre in North India. Indian J Med Res 146:642–645
Makroo RN, Hegde V, Chowdhry M, Bhatia A, Rosamma NL (2015) Seropevalence of infectious markers and their trends in blood donors in a hospital based blood bank in North India. Indian J Med Res 142(3):317–322
Laperche S (2005) Blood safety and nucleic acid testing in Europe (editorial). Eurosurveillance 10(2):1–2
Stramer SL (2002) US NAT yield: where are we after 2 years? Transfus Med 12:243–253
Transfusion Transmissible Infections in Australia:2014 Surveillance report from the Kirby Institute, The University of New South Wales and Australian Red Cross Blood Service 2014. Available at http://kirby.unsw.edu.au/sites/default/files/kirby/report/SERP_2014-Australian-Blood - Donors-Surveillance. Accessed from 10 Nov 2020
Kalra A (2020) Evaluation of a sensitive Multiplex PCR based Minipool NAT for donor blood screening: a single centre experience in Punjab India. Int J Med ScCurr Res 3(2):310–315
On behalf of the ISBT TTI working party, “HBV safety study-group”. Characterisation of Occult HBV yield cases identified by NAT ( ID or small pool) or anti- HBc screening. http://www.isbtweb.org/files/documentation/ISBT-HBV-5Accessed Available from 09 Nov 2020
Dhawan HK, Marwaha N, Sharma RR, Chawla Y et al (2008) Anti- HBc screening in Indian blood donors: still an unresolved issue. World J Gastroentrol 34:5327–5330
Ranganathan S, Iyer RN (2007) How close are we to safe blood transfusion- an insight into HBV infection. Indian J Haematol Blood Transfus 33:1–2
Li L, Chen MH, Chak KF, Lin KS, Tsai LSJ (2008) A pilot study for screening blood donors in Taiwan by nucleic acid amplification technology: detecting occult Hepatitis C Virus and B virus infections and closing the serology window period for Hepatitis C virus. Transfusion 48(6):1198–1206
Pandey P, Towari AK, Dara RC et al (2015) A comprehensive serological and supplemental evaluation of hepatitis B seroyield blood donors: a cross sectional study from a tertiary healthcare centre in India. Asian J Transfus Sci 9(2):189–194
Chaurasia R, Rout D, Zaman S et al (2016) Comparison of procleix ultrio elite and procleix ultrio NAT assays for screening of Transfusion Transmitted Infections among Blood donors in India. Int J Microbiol. https://doi.org/10.1155/2016/2543156
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
Conception, design, interpretation: Dr Sudha Ranganathan; Drafting paper: Dr Ranganthan N Iyer; Biostatistics: Dr N Balakrishna.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no disclosures of conflicts of interest.
Informed Consent
Compliant with standards.
Consent for Publication
The authors declare that this work has not been submitted for publication or has been published in any other journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranganathan, S., Iyer, R.N. & Balakrishna, N. Significance of Adopting Nucleic Acid Amplification Technique for Blood Donor Screening in a Resource Limited Setting: A Study from a Single Centre in South India. Indian J Hematol Blood Transfus 38, 571–576 (2022). https://doi.org/10.1007/s12288-021-01500-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-021-01500-2