Abstract
Background
Targeted axillary dissection (TAD) combines sentinel node biopsy (SNB) with the removal of the previously marked metastatic node. TAD is a promising concept for axillary restaging in node-positive breast cancer patients with pathological complete response (pCR) to neoadjuvant therapy (NAT). We aimed to evaluate TAD feasibility in this context.
Methods
A prospective observational study was conducted in biopsy-confirmed cN1 patients. The removal of the clipped node (CN) was guided by intraoperative ultrasound. SNB used indocyanine green and patent blue V dye. If the CN or sentinel lymph nodes (SLN) had any metastatic foci, or the TAD procedure was unsuccessful, the patient underwent axillary lymph node dissection (ALND).
Results
Thirty-seven patients were included. TAD and SNB identification rates were 97.3%. Every retrieved CN was also a SLN. At the individual level, SNB identification rate was 89.2% with indocyanine green and 85.5% with patent blue V dye. The CN identification rate was 81.1%, being higher when the CN was localized on the intraoperative ultrasound (84.4% vs 60.0%). Nodal pCR was achieved by 54.1% of our patients and was more frequent in HER2-positive and triple-negative tumors (p = 0.039). Nineteen patients were spared from ALND.
Conclusion
TAD with intraoperative ultrasound-guided excision of the CN and SNB with indocyanine green and patent blue V dye is a feasible concept to identify patients without axillary residual disease after NAT, that can be spared from ALND, although the need for marking the biopsied node should be further investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of nodal metastasis in breast cancer is an important prognostic factor and axillary lymph node dissection (ALND) is the gold standard treatment for locoregional control in node-positive patients [1, 2]. However, ALND is associated with important morbidity [3]. In patients with locally advanced breast cancer and nodal metastasis, neoadjuvant therapy (NAT) is used to downstage the tumor and increase the likelihood of breast-conserving surgery (BCS). Nevertheless, persistent nodal disease after NAT increases the risk for locoregional recurrence [4,5,6]. Nodal pathological complete response (pCR) rates up to 40–50% can be achieved, even more in HER2-positive and triple-negative tumors [7,8,9,10]. These cases do not benefit from ALND, which raised the possibility of a more conservative surgical approach, with similar oncological outcomes [1, 5, 11].
Sentinel node biopsy (SNB) is an alternative with less morbidity and provides reliable nodal staging information in node-negative patients [3, 12]. However, its application after NAT in initially node-positive patients is controversial due to historically higher false negative rates (FNR) and lower identification rates (IR) [4, 7], explained by the alterations on the lymphatic drainage caused by NAT and residual disease [13]. If this higher FNR translates into a clinically relevant risk of recurrence remains unclear [14]. The dual-mapping technique with technetium-99 m and blue dye improved the IR from 77.4 to 87.8% (p = 0.046) in SENTINA trial [4] and the FNR from 20.3 to 10.8% (p = 0.05) in ACOSOG Z1071 trial [7]. In GANEA 2 study [10], the removal of at least two sentinel lymph nodes (SLN) decreased the FNR from 19.3 to 7.8% (p = 0.041), while use of immunohistochemistry decreased the FNR from 13.3 to 8.4% in SN FNAC study [5].
An alternative to Technetium-99 m is indocyanine green (ICG) and the detection of its near-infrared fluorescence. ICG allows high IR, good visualization of lymphatic pathways and its addition to patent blue V dye (PBD) has similar results to the Technetium-99 m/blue dye combination [6, 15]. The metastatic growth replaces the reticuloendothelial cells that retain the radioisotope and its transporter molecule inside the node, hampering radioisotope efficiency, while ICG is hypothetically unaffected by this mechanism since its lymphatic drainage is passive [16, 17].
Targeted axillary dissection (TAD) concept may be a feasible alternative to SNB in this context. TAD is the sum of SNB with the removal of the previously marked lymph nodes (clipped node biopsy/CNB); the metastatic nodes are marked before NAT and later removed during SNB, since their pathological evaluation can improve the assessment of residual nodal disease after NAT [1, 11]. A positive clipped node (CN) represents disease resistant to systemic therapy, being more significant than in the setting of upfront SNB, where adjuvant therapy will eventually treat residual disease [18]. Caudle et al. [1] found a FNR of 2.0% for TAD, 4.2% for CNB, and 10.1% for SNB. Thus, TAD concept seems to be the most accurate for axillary restaging after NAT [2], particularly when the CN is also a SLN, although the concordance rate is not always 100%. In ACOSOG Z1071 trial [11], the SNB FNR was 19.0% when the CN was within the ALND specimen, 14.3% if the clip was not found, 13.4% if a clip was not placed, but only 6.8% when the CN was also a SLN.
Our primary aim was to evaluate the feasibility of TAD—CNB with intraoperative ultrasound (IOUS) and SNB with ICG and PBD—in the axillary restaging after NAT of initially node-positive patients. We also aimed to evaluate the concordance between the CN and the SLN, nodal pCR to NAT and the ALND avoidance rate.
Patients and methods
This prospective, phase 1–2, observational single-center study was conducted between November 2019 and September 2021 in the Breast Center of Centro Hospitalar Universitário São João. The study was approved by the Hospital Ethics Committee and written informed consent was obtained from all patients. Data were collected during surgery and from clinical records, radiology, and pathology reports. All medical and surgical treatments and the eligibility for NAT and TAD were decided by a multidisciplinary tumor board.
Eligibility criteria
Female patients with invasive breast cancer submitted to systemic NAT, that were biopsy-proven cN1 before NAT and converted to ycN0 after NAT based on physical examination, were consecutively recruited. Patients with cN0, cN2 or cN3 stage, distant metastasis at diagnosis, inflammatory carcinoma or previous axillary surgery were excluded. Patients with three or more suspicious nodes on the initial ultrasound directly underwent ALND after NAT and were also excluded.
Suspicious lymph nodes marking
All patients underwent ultrasonography for initial axillary staging and lymph node biopsy by fine-needle aspiration or core-needle biopsy. Suspicious nodes were marked with an echo-visible metal clip (HydroMARK®) at the same time of lymph node biopsy, before NAT. A maximum of two clips were placed by patient. When only the most suspicious node was marked, the radiologist referenced the CN spatial relationships with other suspicious nodes, which would be given particular attention during surgery.
NAT and adjuvant therapy
NAT consisted of chemotherapy or endocrine therapy. In HER2-positive tumors, trastuzumab and pertuzumab were added. After completion of NAT, the clinical response of the tumor and axilla was evaluated by physical examination, ultrasound, mammography, and magnetic resonance imaging (MRI). Patients were then submitted to BCS or mastectomy and TAD. Based on tumor characteristics and residual disease, adjuvant breast radiotherapy, regional nodal irradiation (RNI), chemotherapy, anti-HER2 agents and endocrine therapy could be used.
Surgical procedure
Careful examination with axillary IOUS was performed immediately after anesthetic induction to visualize the clip. The CN was selectively removed and radiographed to confirm the clip presence. SNB was done with a combined technique using a peritumoral injection of 1 cc of ICG (5 mg) and a sub-areolar injection (Sappey plexus) of 1 cc of PBD. ICG lymphatic drainage was traced by fluorescence navigation with a near-infrared camera and real-time screen visualization. A node was considered a SLN if it was blue, fluorescent, or clinically suspicious on palpation. The pathological status of every node was determined intraoperatively with One Step Nucleic Acid Amplification or conventional hematoxylin–eosin assay. A node was considered positive if it had any metastatic focus, including micrometastasis and isolated tumor cells. If the CN or SLN was positive after NAT, or TAD surgery was unsuccessful, ALND was performed.
Statistical analysis
SNB was considered effective if it at least one SLN was removed and CNB was successful if the CN was recovered. TAD was unsuccessful if both SNB and CNB could not recover any node, but effective if at least one lymph node, either SLN or CN, was retrieved. The concordance rate was defined as the proportion of surgically recovered CN that were SLN. Data are expressed as absolute and relative frequencies for categorical variables and median and range (minimum–maximum) for quantitative variables. Fisher’s exact test and Pearson's chi-squared test were used for categorical variables, and Mann–Whitney U test for quantitative variables. Statistical tests were two-sided and p values lower than 0.05 were considered statistically significant. Statistical analysis was done with IBM SPSS Statistics 26.0 software.
Results
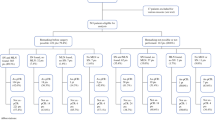
Between April 1, 2019 and April 30, 2021, 645 patients were diagnosed with invasive breast cancer. Among 229 patients undergoing NAT, 37 patients matched our inclusion and exclusion criteria (Fig. 1). Patient demographics and tumor characteristics are listed on Table 1. One patient with three and one patient with four suspicious nodes were included because their most suspicious node was marked with a clip by the Radiology team, and both patients also underwent ALND. Two clips were placed in only one patient.
Flowchart of patients diagnosed with invasive breast cancer between April 1, 2019 and April 30, 2021. NAT neoadjuvant therapy, ALND axillary lymph node dissection, TAD targeted axillary dissection. aOne patient died of unrelated diseases and two patients under neoadjuvant therapy were not submitted to surgery yet
Thirty-six patients received neoadjuvant chemotherapy and one patient endocrine therapy. Fifteen patients also received trastuzumab and pertuzumab. The median NAT duration was four months (range 3–6). Thirteen patients had a complete and 22 patients a partial clinical response in the breast. Thirty-five patients showed nodal response on ultrasound: 17 patients had a complete and 18 patients a partial clinical response. SNB was done with ICG and PBD, except in one patient with an acute allergic reaction to PBD that was reoperated with radioisotope. A median of three SLN (range 1–5) was removed. In all five patients with only one SLN, this was also the previously marked CN, and three of them underwent ALND since the CN was metastatic. Among seven patients with two SLN, there was only one patient without recovery of the CN, who was submitted to ALND since both SLN were metastatic. The CN was identified on the post-NAT ultrasound in 30 patients and with IOUS in 32 patients. At the time of surgery, the CN location was unknown in four cases not undergoing ALND and in two patients with ALND (the ALND specimen was not radiographed, and the clip was not mentioned on the pathology report). Nineteen patients were spared from ALND, and all of them underwent further breast radiotherapy and RNI. Among 18 patients undergoing ALND, the median number of retrieved nodes was ten (range 4–33), metastatic nodes were found on 11 patients and the median number of metastatic nodes found on the ALND specimen was one (range 0–33). Thirty patients underwent BCS and seven total mastectomy (Table 2).
The IR of SNB and TAD was equal, 97.3% (95% CI 91.9–100), because every retrieved CN was a SLN. The IR of CNB was 81.1% (95% CI 68.1–94.1), being higher when the CN was localized on the IOUS, 84.4% vs 60.0% (p = 0.233). Among 7 cases without retrieval of the CN, SNB was effective in six patients and a minimum of three SLN was removed, except in one patient with two metastatic SLN that underwent ALND. It was possible to recover both CN in the patient with two clips; thus, the CNB identification rate considering the total number of clips placed was 81.6%. At the individual level, SNB IR was 89.2% for ICG, 85.5% for PBD and 94.6% for the ICG-PBD combination. In healthy weight patients, ICG and PBD IR were both 92.9%; in overweight patients 91.7% for ICG and 83.3% for PBD; and in obese patients, both IR were 81.8%. At the lymph node level, SNB IR was 77.7% for ICG and 64.1% for PBD (p = 0.085), 88.3% for the ICG-PBD combination and 96.1% when also considering clinically suspicious nodes. Among 103 SLN, 24 were metastatic: six of 25 fluorescent nodes; five of 11 blue nodes; nine of 55 fluorescent and blue nodes; one of four radioisotope-active nodes; and three of eight clinically suspicious nodes (Fig. 2). Besides one acute allergic reaction to PBD, there were no complications during TAD or clip placement.
Among 18 cases undergoing ALND, the SLN and/or CN were the only positive nodes in six cases. The ALND specimen was also negative in the patient with unsuccessful TAD. Within 15 cases with positive retrieved SLN and effective CNB, two cases had a positive SLN not CN: in one patient, the CN had signs of previous metastasis (fibrosis); in the other, the ALND specimen was negative. Metastatic status of SNB, CNB, TAD and ALND specimens are listed on Table 3. Negative status of the recovered CN (p < 0.001), breast pCR (p = 0.006), HER2-positive and triple-negative tumors (p = 0.039) were significantly associated with nodal pCR (Table 4).
Discussion
We present our initial results with TAD concept. More than half of our patients could be spared from ALND. TAD and SNB IR were high (36/37): the only patient with an ineffective TAD was the first patient included. Our CNB IR of 81.1% was lower than some of the previously reported [19, 20]. This may be explained by the procedure learning curve, which also affected results of previous series [21, 22], and must be considered when implementing a new technique.
Clip-based TAD is the technique with more data available. Like the ILINA trial [19], we used IOUS for localizing the CN. This process was sometimes difficult and slow. Occasionally, the clip and its mucoid coating were naked-eye visible during SLN dissection and could be lost inadvertently; thus, the clip must be positioned inside the suspicious node. Metal-hydrogel clips’ visibility on ultrasound may reduce with time [22] and accurate identification may be challenging, especially if the CN reverts to normal after NAT [23]. Still, IOUS has lower costs, is radiation-free, and facilitates surgical scheduling [19, 23].
It was not possible to localize the CN with IOUS in five patients. The CN was recovered in 84.4% of cases with visualization on the IOUS, but only in 60.0% of cases without this visualization, supporting that removal of the CN benefits from its ultrasound visibility and the team experience [2, 18, 23]. In the ILINA trial [19], the CN predicted the axillary status after NAT in 97.1% of cases and the FNR was 4.1%. However, if the clip was not clearly visualized, an attempt was made to place another clip close to the first marker and patients in which the CN could not be localized by imaging studies were excluded, which did not happen in our study and may explain our lower CNB IR. Non-recovery of the CN may also have been due to clip dislodgement during SLN/CN dissection [22, 24], since in one of our patients the clip was found inside a seroma on a post-surgery ultrasound.
Presurgical ultrasound-guided wire localization of the CN had high IR in some studies [9, 20, 24], but results are not always satisfactory [21], it requires an additional invasive procedure; displacement and discomfort are possible, and some radiologists are concerned with the proximity to axillary vessels [22, 25]. Preoperative localization of the CN with seeds [13] or ink [26] may also improve removal of the CN. Other alternative is tattooing positive nodes with a carbon suspension at the time of diagnosis [27], a low-cost technique without invasive localization procedures, but limitations include ink scattering to other nodes, difficult distinction between the blue dye and black ink, and the need for a wider surgical dissection [23, 27, 28]. Another option is the MARI (Marking Axillary lymph nodes with Radioactive Iodine seeds) procedure, which uses 125I seeds that facilitate surgical scheduling and have a decreased risk of displacement and injury to vascular structures but are more expensive and limited by some country’s regulations [23, 28, 29]. A non-radioactive option is ferromagnetic seed, which implies a higher cost and the possibility of MRI artifacts [23, 28, 30].
Markers can be placed at the same time of percutaneous node biopsy, as in our study, which avoids the need for an additional procedure and assures the marker has been placed into the biopsied node but has the risk of placement in negative nodes; or during a second invasive procedure after the biopsy results show metastasis, although the marker may not be placed into the previously biopsied node [23, 25]. In most studies, only one node is marked, although if it is the largest, the most suspicious, or the most accessible is variable and other features besides size should be considered [31]. This option is less expensive, but the number of metastatic nodes at diagnosis is unknown and initial ultrasound may not detect all nodes needed to be marked [23]. Since different nodes may have heterogenous responses to NAT and other nodes beyond the CN may have residual disease, mark all metastatic nodes that can more accurately predict axillary status and reduce FNR [23, 32].
The meta-analysis by Simons et al. [33] found a negative predictive value up to 97% for TAD: when nodal pCR is predicted, residual disease is missed in few patients. Several studies supported the combination of SNB with CNB [1, 24, 33]. The added value of SNB in the TAD procedure was also proved by our results, since three cases with a positive ALND specimen were only detected by SNB; in six cases with ineffective CNB, SNB was successful in staging the axilla; and even if it is not possible to localize the CN, we may surgically recover it as a SLN [22]. In the study by Caudle et al. [1], the only factor associated with the discordance between the CN and the SLN was the presence of at least four abnormal nodes on the initial ultrasound. However, we mostly included patients with one or two abnormal nodes, which can partly explain our concordance rate of 100%. Since every retrieved CN was also a SLN, this could mean that, when a SNB combined technique with ICG is used, CNB could be avoided.
We found a SNB IR higher than some of the previously reported [1, 4], which can be explained by the dual-mapping technique with ICG and PBD. After NAT, the axilla has more fibrosis and the lymphatic drainage from the breast can be impaired, thus a dual-mapping technique is highly recommended [4, 7]. Even though removal of more SLN is associated with lower FNR [34], it is also important to remove as few nodes as possible to accomplish the primary aim of reducing morbidity [33]. In our cohort, it was possible to remove at least three SLN in most cases; in five cases with a single retrieved SLN, this was the previously marked CN; among seven cases with two SLN, one of the SLN was the CN, except in one patient without CN recovery who underwent ALND. Thus, since at least three SLN or the CN were removed, we believe it was safe to omit ALND in those patients with non-metastatic TAD nodes.
Contrary to most studies, which used an association of Technetium-99 m and blue dye, we combined ICG with PBD. ICG has less cost, logistical challenges, and an easier real-time visualization [15, 25]. However, its efficiency may be limited by high BMI [35], which was also demonstrated by our results. Chirappapha et al. [6] found higher IR and lower FNR for ICG comparing with isosulfan blue or radioisotope, in N + patients, after NAT. In their study, the combination of ICG and blue dye was associated with the highest IR and was significantly better than the blue dye-radioisotope combination (p = 0.014). In line with our results, this supports the feasibility of combining PBD with ICG after NAT.
Damin et al. [36] found that, among node-positive patients undergoing NAT, axillary recurrence was similar (p = 0.71) in patients only offered SNB with Technetium-99 m and PBD, and patients submitted to ALND. Overall survival, disease-free survival and distant recurrence were significantly better in patients only submitted to SNB, since they achieved nodal pCR. Axillary recurrence does not seem to depend on the type of axillary surgery [14], which supports the use of SNB or TAD in this context.
Although some studies developed predictive models of nodal pCR after NAT [37, 38], to select who may not require ALND is challenging, since clinical and imaging exams are limited in distinguishing residual disease from nodal pCR [5, 19, 29]. In our study, axillary clinical response, based on imaging exams, did not significantly predict nodal pCR (p = 0.201). Our nodal pCR rate was 54.1% and our findings support that, in addition to TAD results, breast pCR and tumor subtype can help us to decide which patients may be safely spared from ALND. Most of our patients received systemic adjuvant therapies and all received RNI, that may eliminate residual disease. Since SNB with RNI has a lower risk of lymphedema than ALND [12], current trials will determine the oncological safety of exchanging ALND for RNI.
Our study has some limitations. The main one is the reduced size of our sample; nevertheless, it represents the practice of a general teaching hospital breast center that manages 350–400 new breast cancer cases per year and was deeply affected by the COVID-19 pandemic. It also represents the learning curve of both surgeons and radiologists in the TAD concept. This was particularly felt during clip placement, CN localization with IOUS and its surgical dissection. Since ALND was only done if the SLN and/or CN were metastatic, or if TAD was unsuccessful, we were not able to evaluate FNR. Exact clip location, which is crucial to evaluate for residual disease in the CN, was unknown in six cases at the time of surgery. However, this study was done in our regular clinical practice, which reinforces its external validity.
Our study supports that TAD concept can detect patients without residual nodal disease after NAT, sparing them from unnecessary ALND. We used a simple, safe, highly effective, and reproducible technique to mark the biopsied node and to identify the CN and SLN. This technique uses passive vital dyes with high IR, implies less costs and logistical challenges. However, according to our results, the real need for marking the biopsied node should be further investigated since, in our cohort, the CN had no added value. Further studies with larger samples and longer follow-up periods should focus on survival, locoregional recurrence and quality of life when ALND is omitted.
References
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–8. https://doi.org/10.1200/JCO.2015.64.0094.
Flores-Funes D, Aguilar-Jimenez J, Martinez-Galvez M, Ibanez-Ibanez MJ, Carrasco-Gonzalez L, Gil-Izquierdo JI, et al. Feasibility and validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: definitive results. Surg Oncol. 2021;38: 101636. https://doi.org/10.1016/j.suronc.2021.101636.
Fougo JL, Dinis-Ribeiro M, Araujo C, Dias T, Reis P, Giesteira L, et al. Impact of lymphadenectomy on axillary recurrence and morbidity of the upper limb in breast cancer patients with negative sentinel node. A prospective randomised study. Cir Esp. 2011;89:307–16. https://doi.org/10.1016/j.ciresp.2011.01.011.
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18. https://doi.org/10.1016/S1470-2045(13)70166-9.
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33:258–64. https://doi.org/10.1200/JCO.2014.55.7827.
Chirappapha P, Chatmongkonwat T, Lertsithichai P, Pipatsakulroj W, Sritara C, Sukarayothin T. Sentinel lymph node biopsy after neoadjuvant treatment of breast cancer using blue dye, radioisotope, and indocyanine green: prospective cohort study. Ann Med Surg (Lond). 2020;59:156–60. https://doi.org/10.1016/j.amsu.2020.09.030.
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61. https://doi.org/10.1001/jama.2013.278932.
Flores-Funes D, Aguilar-Jimenez J, Martinez-Galvez M, Ibanez-Ibanez MJ, Carrasco-Gonzalez L, Gil-Izquierdo JI, et al. Validation of the targeted axillary dissection technique in the axillary staging of breast cancer after neoadjuvant therapy: preliminary results. Surg Oncol. 2019;30:52–7. https://doi.org/10.1016/j.suronc.2019.05.019.
Garcia-Novoa A, Acea-Nebril B, Diaz Carballada C, Bouzon Alejandro A, Conde C, Cereijo Garea C, et al. Combining wire localization of clipped nodes with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer: preliminary results from a prospective study. Ann Surg Oncol. 2021;28:958–67. https://doi.org/10.1245/s10434-020-08925-5.
Classe JM, Loaec C, Gimbergues P, Alran S, de Lara CT, Dupre PF, et al. Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients: the GANEA 2 study. Breast Cancer Res Treat. 2019;173:343–52. https://doi.org/10.1007/s10549-018-5004-7.
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263:802–7. https://doi.org/10.1097/SLA.0000000000001375.
Naoum GE, Roberts S, Brunelle CL, Shui AM, Salama L, Daniell K, et al. Quantifying the impact of axillary surgery and nodal irradiation on breast cancer-related lymphedema and local tumor control: long-term results from a prospective screening trial. J Clin Oncol. 2020;38:3430–8. https://doi.org/10.1200/JCO.20.00459.
Simons JM, van Pelt M, Marinelli A, Straver ME, Zeillemaker AM, Pereira Arias-Bouda LM, et al. Excision of both pretreatment marked positive nodes and sentinel nodes improves axillary staging after neoadjuvant systemic therapy in breast cancer. Br J Surg. 2019;106:1632–9. https://doi.org/10.1002/bjs.11320.
Kahler-Ribeiro-Fontana S, Pagan E, Magnoni F, Vicini E, Morigi C, Corso G, et al. Long-term standard sentinel node biopsy after neoadjuvant treatment in breast cancer: a single institution ten-year follow-up. Eur J Surg Oncol. 2021;47:804–12. https://doi.org/10.1016/j.ejso.2020.10.014.
Tsuyuki S, Yamaguchi A, Kawata Y, Kawaguchi K. Assessing the effects of neoadjuvant chemotherapy on lymphatic pathways to sentinel lymph nodes in cases of breast cancer: usefulness of the indocyanine green-fluorescence method. Breast. 2015;24:298–301. https://doi.org/10.1016/j.breast.2015.02.034.
Tausch C, Baege A, Rageth C. Mapping lymph nodes in cancer management—role of (99m)Tc-tilmanocept injection. Onco Targets Ther. 2014;7:1151–8. https://doi.org/10.2147/OTT.S50394.
Borgstein PJ, Pijpers R, Comans EF, van Diest PJ, Boom RP, Meijer S. Sentinel lymph node biopsy in breast cancer: guidelines and pitfalls of lymphoscintigraphy and gamma probe detection. J Am Coll Surg. 1998;186:275–83. https://doi.org/10.1016/s1072-7515(98)00011-8.
Kanesalingam K, Sriram N, Heilat G, Ng EE, Meybodi F, Elder E, et al. Targeted axillary dissection after neoadjuvant systemic therapy in patients with node-positive breast cancer. ANZ J Surg. 2020;90:332–8. https://doi.org/10.1111/ans.15604.
Siso C, de Torres J, Esgueva-Colmenarejo A, Espinosa-Bravo M, Rus N, Cordoba O, et al. Intraoperative ultrasound-guided excision of axillary clip in patients with node-positive breast cancer treated with neoadjuvant therapy (ILINA Trial): a new tool to guide the excision of the clipped node after neoadjuvant treatment. Ann Surg Oncol. 2018;25:784–91. https://doi.org/10.1245/s10434-017-6270-z.
Kim EY, Byon WS, Lee KH, Yun JS, Park YL, Park CH, et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: a preliminary study. World J Surg. 2018;42:582–9. https://doi.org/10.1007/s00268-017-4171-8.
Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018;44:1307–11. https://doi.org/10.1016/j.ejso.2018.05.035.
Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol. 2017;24:3011–6. https://doi.org/10.1245/s10434-017-6023-z.
Banys-Paluchowski M, Gasparri ML, de Boniface J, Gentilini O, Stickeler E, Hartmann S, et al. Surgical management of the axilla in clinically node-positive breast cancer patients converting to clinical node negativity through neoadjuvant chemotherapy: current status, knowledge gaps, and rationale for the EUBREAST-03 AXSANA Study. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13071565.
Gurleyik G, Aksu SA, Aker F, Tekyol KK, Tanrikulu E, Gurleyik E. Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy. Ann Surg Treat Res. 2021;100:305–12. https://doi.org/10.4174/astr.2021.100.6.305.
Ersoy YE, Kadioglu H. Review of novel sentinel lymph node biopsy techniques in breast cancer patients treated with neoadjuvant chemotherapy. Clin Breast Cancer. 2018;18:e555–9. https://doi.org/10.1016/j.clbc.2018.01.004.
Pinto D, Batista E, Gouveia P, Mavioso C, Anacleto J, Ribeiro J, et al. Targeted axillary dissection after chemotherapy: feasibility study with clip and carbon dye tattoo—neotarget trial. Breast Care. 2021. https://doi.org/10.1159/000517208.
Natsiopoulos I, Intzes S, Liappis T, Zarampoukas K, Zarampoukas T, Zacharopoulou V, et al. Axillary lymph node tattooing and targeted axillary dissection in breast cancer patients who presented as cN+ before neoadjuvant chemotherapy and became cN0 after treatment. Clin Breast Cancer. 2019;19:208–15. https://doi.org/10.1016/j.clbc.2019.01.013.
Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13071539.
Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261:378–82. https://doi.org/10.1097/SLA.0000000000000558.
Greenwood HI, Wong JM, Mukhtar RA, Alvarado MD, Price ER. Feasibility of magnetic seeds for preoperative localization of axillary lymph nodes in breast cancer treatment. AJR Am J Roentgenol. 2019;213:953–7. https://doi.org/10.2214/AJR.19.21378.
Cabioglu N, Karanlik H, Kangal D, Ozkurt E, Oner G, Sezen F, et al. Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25:3030–6. https://doi.org/10.1245/s10434-018-6575-6.
Lim GH, Gudi M, Teo SY, Ng RP, Yan Z, Lee YS, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy? Oncologist. 2020;25:e1621–7. https://doi.org/10.1634/theoncologist.2020-0494.
Simons JM, van Nijnatten TJA, van der Pol CC, Luiten EJT, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019;269:432–42. https://doi.org/10.1097/SLA.0000000000003075.
Tee SR, Devane LA, Evoy D, Rothwell J, Geraghty J, Prichard RS, et al. Meta-analysis of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with initial biopsy-proven node-positive breast cancer. Br J Surg. 2018;105:1541–52. https://doi.org/10.1002/bjs.10986.
Grischke EM, Rohm C, Hahn M, Helms G, Brucker S, Wallwiener D. ICG fluorescence technique for the detection of sentinel lymph nodes in breast cancer: results of a prospective open-label clinical trial. Geburtshilfe Frauenheilkd. 2015;75:935–40. https://doi.org/10.1055/s-0035-1557905.
Damin AP, Zancan M, Melo MP, Biazus JV. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with node-positive breast cancer: guiding a more selective axillary approach. Breast Cancer Res Treat. 2021;186:527–34. https://doi.org/10.1007/s10549-020-06011-8.
Kantor O, Sipsy LM, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018;25:1304–11. https://doi.org/10.1245/s10434-018-6345-5.
Flores-Funes D, Aguilar-Jimenez J, Martinez-Galvez M, Ibanez-Ibanez MJ, Carrasco-Gonzalez L, Gil-Izquierdo JI, et al. Development of a predictive score of axillary lymph node dissection based on targeted axillary dissection in patients with breast cancer diagnosis, affected lymph nodes, and neoadjuvant treatment. Surg Oncol. 2021;38: 101629. https://doi.org/10.1016/j.suronc.2021.101629.
Funding
The authors declare they have received no financial assistance.
Author information
Authors and Affiliations
Contributions
CSP: Data collection, analysis and interpretation, statistical analysis, manuscript preparation and editing and approval of the final manuscript. JLF: Study design, surgical procedures, data collection, manuscript review, supervision, and approval of the final manuscript. BP: Statistical analysis, manuscript review and approval of the final manuscript. CAP: Manuscript review and approval of the final manuscript. FO, SC, AM, HM, JA, DG: Surgical procedures, data collection and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Centro Hospitalar Universitário São João Ethics Committee (CE 323-19).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Pinto, C.S., Peleteiro, B., Pinto, C.A. et al. Initial experience with targeted axillary dissection after neoadjuvant therapy in breast cancer patients. Breast Cancer 29, 709–719 (2022). https://doi.org/10.1007/s12282-022-01349-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01349-x