Abstract
Background
Triple negative breast cancer (TNBC) is the most aggressive subtype with the worst prognosis. The role of profilin 2 (PFN2) in TNBC is very controversial. The current study is to explore the role of PFN2 in TNBC.
Methods
PFN2 expression in TNBC and normal breast tissues were evaluated by immunohistochemical analysis. The association between PFN2 expression and prognosis in TNBC patients was analyzed from the TCGA database. A cell counting kit-8 (CCK8) assay was employed to investigate the effects of PFN2 in TNBC cell proliferations. The migration and invasion capability of TNBC cells was evaluated by transwell assays. Western blot was performed to assess the related protein expression of TGF-β/Smad signaling and epithelial to mesenchymal transition. Finally, TNBC xenografts were established to determine the tumorigenicity in vivo using female Nod/Scid mice.
Results
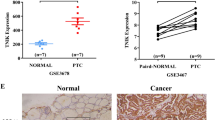
PFN2 is upregulated in TNBC and the higher expression was associated with worse survival. CCK8 assays and Transwell assays demonstrated that PFN2 promoted the proliferation, migration and invasion of TNBC cells. Smad2 and Smad3 were upregulated in PFN2 overexpressing TNBC cells, which further induced the process of epithelial‑to‑mesenchymal transition. Similarly, the overexpressing PFN2 TNBC cells exhibited stronger tumorigenicity in vivo.
Conclusions
Higher PFN2 expression is associated with a worse 10-year overall survival and relapse-free survival in breast cancer patients, as well as worse 10-year relapse-free survival in TNBC patients. PFN2 promotes the proliferation, migration and invasion of TNBC cells by regulating epithelial-to-mesenchymal transition.





Similar content being viewed by others
References
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48.
Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat. 2017;161:279–87.
Zeichner SB, Terawaki H, Gogineni K. A review of systemic treatment in metastatic triple-negative breast cancer. Breast Cancer (Auckl). 2016;10:25–36.
Li Y, Grenklo S, Higgins T, et al. The profilin:actin complex localizes to sites of dynamic actin polymerization at the leading edge of migrating cells and pathogen-induced actin tails. Eur J Cell Biol. 2008;87:893–904.
Yan J, Ma C, Gao Y. MicroRNA-30a-5p suppresses epithelial-mesenchymal transition by targeting profilin-2 in high invasive non-small cell lung cancer cell lines. Oncol Rep. 2017;37:3146–54.
Tang YN, Ding WQ, Guo XJ, et al. Epigenetic regulation of Smad2 and Smad3 by profilin-2 promotes lung cancer growth and metastasis. Nat Commun. 2015;6:8230.
Zhang H, Yang W, Yan J, et al. Loss of profilin 2 contributes to enhanced epithelial-mesenchymal transition and metastasis of colorectal cancer. Int J Oncol. 2018;53:1118–28.
Kim MJ, Lee YS, Han GY, et al. Profilin 2 promotes migration, invasion, and stemness of HT29 human colorectal cancer stem cells. Biosci Biotechnol Biochem. 2015;79:1438–46.
Ma CY, Zhang CP, Zhong LP, et al. Decreased expression of profilin 2 in oral squamous cell carcinoma and its clinicopathological implications. Oncol Rep. 2011;26:813–23.
Cui XB, Zhang SM, Xu YX, et al. PFN2, a novel marker of unfavorable prognosis, is a potential therapeutic target involved in esophageal squamous cell carcinoma. J Transl Med. 2016;14:137.
Liu J, Wu Y, Wang Q, et al. Bioinformatic analysis of PFN2 dysregulation and its prognostic value in head and neck squamous carcinoma. Future Oncol. 2018;14:449–59.
Zhou K, Chen J, Wu J, et al. Profilin 2 promotes proliferation and metastasis of head and neck cancer cells by regulating PI3K/AKT/beta-catenin signaling pathway. Oncol Res. 2019;27:1079–88.
Liu Y, Li H, Liu F, et al. Heterogeneous nuclear ribonucleoprotein A2/B1 is a negative regulator of human breast cancer metastasis by maintaining the balance of multiple genes and pathways. EBioMedicine. 2020;51:102583.
Mouneimne G, Hansen SD, Selfors LM, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22:615–30.
Jiang M, Qiu N, Xia H, et al. Long noncoding RNA FOXD2AS1/miR1505p/PFN2 axis regulates breast cancer malignancy and tumorigenesis. Int J Oncol. 2019;54:1043–52.
Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–20.
Massague J. TGFbeta in cancer. Cell. 2008;134:215–30.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–72.
Acknowledgements
We are in debt to Dr. Chunjie Wang and Dr. Nick Baniak (Department of Pathology and Laboratory Medicine, College of Medicine, University of Saskatchewan, Saskatoon, Canada) for reviewing the manuscript and providing professional input.
Funding
This work was supported by the Beijing Municipal Health System Academic Leaders of High-level Health Personnel Program, P.R. China (No. 2011-2-28) and Beijing Breast Disease Prevention and Treatment Society, Cancer Prevention and Treatment Research Projects, P.R. China (No. 2015-8-8).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was waived because of the nature of retrospective study, and the patient data were kept confidentially. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ling, Y., Cao, Q., Liu, Y. et al. Profilin 2 (PFN2) promotes the proliferation, migration, invasion and epithelial-to-mesenchymal transition of triple negative breast cancer cells. Breast Cancer 28, 368–378 (2021). https://doi.org/10.1007/s12282-020-01169-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01169-x