Abstract
Purpose of Review
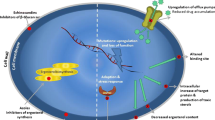
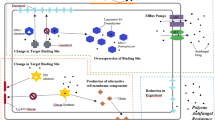
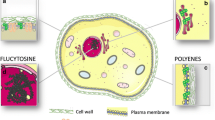
This review summarizes current treatment options for echinocandin-resistant Candida spp. (ERC) and azole-resistant Aspergillus fumigatus (ARAF), emphasizing recent in vitro/in vivo data, clinical reports, and consensus statements.
Recent Findings
Advances in ERC and ARAF treatment are limited to specific antifungal combinations and dose optimization but remain reliant on amphotericin products. Although novel antifungals may provide breakthroughs in the treatment of resistant fungi, these agents are not yet available. Early identification and appropriate treatment remain a paramount, albeit elusive, task.
Summary
When either ERC or ARAF are suspected or proven, amphotericin products remain the cornerstone of initial therapy. For ERC, azoles are de-escalation options for susceptible isolates in stable patients to avoid amphotericin toxicities. Although combination echinocandin with high-dose salvage posaconazole or isavuconazole may be attempted in ARAF, it requires careful consideration following patient stabilization. Future research defining optimal therapies and early identification of ERC and ARAF is of extreme importance.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. https://doi.org/10.1111/myc.12330.
Perlin DS. Echinocandin resistance in Candida. Clin Infect Dis. 2015;61:S612–7. https://doi.org/10.1093/cid/civ791.
Matzaraki V, Gresnigt MS, Jaeger M, Ricaño-Ponce I, Johnson MD, Oosting M, et al. An integrative genomics approach identifies novel pathways that influence candidaemia susceptibility. PLoS One. 2017;12:e0180824. https://doi.org/10.1371/journal.pone.0180824.
•• Verweij PE, Ananda-Rajah M, Andes D, Arendrup MC, Brüggemann RJ, Chowdhary A, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat. 2015;21–22:30–40 https://linkinghub.elsevier.com/retrieve/pii/S1368764615000357. Accessed October 30, 2019 Expert consensus statement focused specifically on the empiric and definitive treatment of azole-resistantAspergillus fumigatus.
Wiederhold N. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017;10:249–59. https://doi.org/10.1016/j.drup.2015.08.001.
Pais P, Galocha M, Viana R, Cavalheiro M, Pereira D, Teixeira MC. Microevolution of the pathogenic yeasts Candida albicans and Candida glabrata during antifungal therapy and host infection. Microb Cell. 2019;6:142–59. https://doi.org/10.15698/mic2019.03.670.
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2015;civ933. https://doi.org/10.1093/cid/civ933
Arendrup MC, Patterson TF. Multidrug-resistant candida: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216:S445–51. https://doi.org/10.1093/infdis/jix131.
Vallabhaneni S, Cleveland AA, Farley MM, Harrison LH, Schaffner W, Beldavs ZG, et al. Epidemiology and risk factors for echinocandin nonsusceptible Candida glabrata bloodstream infections: Data from a large multisite population-based candidemia surveillance program, 2008–2014. Open Forum Infect Dis. 2015;2(4):ofv163. https://doi.org/10.1093/ofid/ofv163
• Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty years of the sentry antifungal surveillance program: results for Candida species from 1997–2016. Open Forum Infect Dis. 2019;6:S79–94. https://doi.org/10.1093/ofid/ofy358Recent comprehensive review for Candida spp. resistance patterns over a period of 20 years.
Centers for Disease Control and Prevention. Recommendation for identification of Candida auris. https://www.cdc.gov/fungal/candida-auris/index.html. Accessed November 1, 2019.
Public Health England. Guidance for the laboratory investigation, management and infection prevention and control for cases of Candida auris. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/637685/Updated_Candida_auris_Guidance_v2.pdf. Accessed November 1, 2019.
Hou X, Lee A, Jiménez-Ortigosa C, Kordalewska M, Perlin DS, Zhao Y. Rapid detection of erg11 -associated azole resistance and fks -associated echinocandin resistance in Candida auris. Antimicrob Agents Chemother. 2018;63:e01811–8. https://doi.org/10.1128/AAC.01811-18.
Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, et al. Development of high-level echinocandin resistance in a patient with recurrent Candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis. 2019;6:ofz262. https://doi.org/10.1093/ofid/ofz262/5510065
•• Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. Comparison of EUCAST and CLSI reference microdilution mics of eight antifungal compounds for candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother. 2017;61:e00485–17. https://doi.org/10.1128/AAC.00485-17Recent publication ofCandida aurisMICs to interpret the extent of resistance although no clinical breakpoints have been set.
Rivero-Menendez O, Navarro-Rodriguez P, Bernal-Martinez L, Martin-Cano G, Lopez-Perez L, Sanchez-Romero I, et al. Clinical and laboratory development of echinocandin resistance in Candida glabrata: Molecular characterization. Front Microbiol. 2019;10:1585. https://doi.org/10.3389/fmicb.2019.01585.
Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, et al. Role of FKS mutations in Candida glabrata: mic values, echinocandin resistance, and multidrug resistance. Antimicrob Agents Chemother. 2014;58:4690–6. https://doi.org/10.1128/AAC.03255-14.
Bordallo-Cardona MÁ, Escribano P, de la Pedrosa GGE, Marcos-Zambrano LJ, Cantón R, Bouza E, et al. In vitro exposure to increasing micafungin concentrations easily promotes echinocandin resistance in Candida glabrata isolates. Antimicrob Agents Chemother. 2016;61(2):e01542–16. https://doi.org/10.1128/AAC.01542-16.
Beardsley J, Halliday CL, Chen SC-A, Sorrell TC. Responding to the emergence of antifungal drug resistance: Perspectives from the bench and the bedside. Future Microbiol. 2018;13:1175–91. https://doi.org/10.2217/fmb-2018-0059.
Jensen RH, Astvad KMT, Silva LV, Sanglard D, Jørgensen R, Nielsen KF, et al. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J Antimicrob Chemother. 2015;70:2551–5. https://doi.org/10.1093/jac/dkv140.
Staab JF, Neofytos D, Rhee P, Jiménez-Ortigosa C, Zhang SX, Perlin DS, et al. Target enzyme mutations confer differential echinocandin susceptibilities in Candida kefyr. Antimicrob Agents Chemother. 2014;58:5421–7. https://doi.org/10.1128/AAC.00096-14.
Healey KR, Jimenez Ortigosa C, Shor E, Perlin DS. Genetic drivers of multidrug resistance in Candida glabrata. Front Microbiol. 2016;7. https://doi.org/10.3389/fmicb.2016.01995.
Healey K, Perlin DS. Fungal resistance to echinocandins and the MDR phenomenon in Candida glabrata. J Fungi. 2018;4(3). https://doi.org/10.3390/jof4030105.
Basas J, Palau M, Gomis X, Almirante B, Gavaldà J. Efficacy of liposomal amphotericin B and anidulafungin using an antifungal lock technique (ALT) for catheter-related Candida albicans and Candida glabrata infections in an experimental model. Coste AT, editor. PLoS One. 2019;14(2):e0212426. https://doi.org/10.1371/journal.pone.0212426
Chew KL, Octavia S, Lin RTP, Yan GZ, Teo JWP. Delay in effective therapy in anidulafungin-resistant Candida tropicalis fungaemia: Potential for rapid prediction of antifungal resistance with whole-genome-sequencing. J Glob Antimicrob Resist. 2019;16:105–7. https://doi.org/10.1016/j.jgar.2018.12.010.
Kullberg BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: A randomised non-inferiority trial. Lancet. 2005;366(9495):1435–42. https://doi.org/10.1016/S0140-6736(05)67490-9.
Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, et al. Increasing echinocandin resistance in Candida glabrata: Clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–32. https://doi.org/10.1093/cid/cit136.
•• Denardi LB, Keller JT, Oliveira V, DAN M, Santurio JM, Alves SH. Activity of combined antifungal agents against multidrug-resistant Candida glabrata strains. Mycopathologia. 2017;182:819–28. https://doi.org/10.1007/s11046-017-0141-9Recent checkerboard analysis of combined antifungal agentsin vitroto predict the synergistic effect againstC. glabrata.
Agnelli C, Guinea J, Valerio M, Escribano P, Emilio B, Munoz P. Infectious endocarditis caused by Candida glabrata: Evidence of in vivo development of echinocandin resistance. Rev Esp Quimioter. 2019;34:395–7.
Wright WF, Bejou N, Shields RK, Marr K, McCarty TP, Pappas PG. Amphotericin B induction with voriconazole consolidation as salvage therapy for FKS - associated echinocandin resistance in Candida glabrata septic arthritis and osteomyelitis. Antimicrob Agents Chemother. 2019;63:e00512–9. https://doi.org/10.1128/AAC.00512-19.
Chowdhary A, Sharma C, Meis JF. Candida auris: A rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. Hogan DA, editor. PLoS Pathog. 2017;13(5):e1006290. https://doi.org/10.1371/journal.ppat.1006290
Rhodes J, Abdolrasouli A, Farrer RA, Cuomo CA, Aanensen DM, Armstrong-James D, et al. Genomic epidemiology of the UK outbreak of the emerging human fungal pathogen Candida auris. Emerg Microbes Infect. 2018;7(1):1–12. https://doi.org/10.1038/s41426-018-0045-x.
Centers for Disease Control and Prevention. Recommendation for identification of Candida auris. https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html. Accessed November 14, 2019.
Fakhim H, Chowdhary A, Prakash A, Vaezi A, Dannaoui E, Meis JF, et al. In vitro interactions of echinocandins with triazoles against multidrug-resistant Candida auris. Antimicrob Agents Chemother. 2017;61:e01056–17. https://doi.org/10.1128/AAC.01056-17.
• Chowdhary A, Sharma C, Meis JF. Azole-resistant aspergillosis: epidemiology, molecular mechanisms, and treatment. J Infect Dis. 2017;216:S436–44. https://doi.org/10.1093/infdis/jix210Recent comprehensive epidemiology review for azole-resistantAspergillus fumigatus.
Denning DW, Venkateswarlu K, Oakley KL, Anderson MJ, Manning NJ, Stevens DA, et al. Itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1364–8. https://doi.org/10.1128/AAC.41.6.1364.
Van der Linden JWM, Arendrup MC, Warris A, Lagrou K, Pelloux H, Hauser PM, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21(6):1041–4. https://doi.org/10.3201/eid2106.140717.
Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in aspergillus fumigatus isolates from the artemis global surveillance study is primarily due to the TR/l98H mutation in the cyp51A Gene. Antimicrob Agents Chemother. 2011;55:4465–8. https://doi.org/10.1128/AAC.00185-11.
Pham CD, Reiss E, Hagen F, Meis JF, Lockhart SR. Passive surveillance for azole-resistant Aspergillus fumigatus, United States, 2011–2013. Emerg Infect Dis. 2014;20(9):1498–503. https://doi.org/10.3201/eid2009.140142.
Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole resistance in Aspergillus fumigatus : Can we retain the clinical use of mold-active antifungal azoles? Weinstein RA, editor. Clin Infect Dis. 2016;62:362–8. https://doi.org/10.1093/cid/civ885.
Camps SMT, van der Linden JWM, Li Y, Kuijper EJ, van Dissel JT, Verweij PE, et al. Rapid induction of multiple resistance mechanisms in aspergillus fumigatus during azole therapy: A case study and review of the literature. Antimicrob Agents Chemother. 2012;56:10–6. https://doi.org/10.1128/AAC.05088-11.
Snelders E, Camps SMT, Karawajczyk A, Schaftenaar G, Kema GHJ, van der Lee HA, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. Feldmesser M, editor. PLoS One. 2012;7(3):e31801. https://doi.org/10.1371/journal.pone.0031801
Van der Linden JWM, Snelders E, Kampinga GA, Rijnders BJA, Mattsson E, Debets-Ossenkopp YJ, et al. Clinical implications of azole resistance in Aspergillus fumigatus , the Netherlands, 2007–2009. Emerg Infect Dis. 2011;17(10):1846–54. https://doi.org/10.3201/eid1710.110226.
Patterson TF, Thompson GR, Denning DW, Fishman JA, Hadley S, Herbrecht R, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1–60. https://doi.org/10.1093/cid/ciw326.
Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24:e1–38. https://doi.org/10.1016/j.cmi.2018.01.002.
Pérez-Cantero A, López-Fernández L, Guarro-Artigas J, Capilla J. Azole resistance mechanisms in Aspergillus: Update and recent advances. Int J Antimicrob Agents. 2019;105807. https://doi.org/10.1016/j.ijantimicag.2019.09.011.
Arikan-Akdagli S, Ghannoum M, Meis J. Antifungal resistance: specific focus on multidrug resistance in Candida auris and secondary azole resistance in Aspergillus fumigatus. J Fungi. 2018;4(4):129. https://doi.org/10.3390/jof4040129.
Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. 2003;47(3):1120–4. https://doi.org/10.1128/aac.47.3.1120-1124.2003.
Nascimento AM, Goldman GH, Park S, Marras SAE, Delmas G, Oza U, et al. Multiple resistance mechanisms among Aspergillus fumigatus mutants with high-level resistance to itraconazole. Antimicrob Agents Chemother. 2003;47:1719–26. https://doi.org/10.1128/AAC.47.5.1719-1726.2003.
Howard SJ, Webster I, Moore CB, Gardiner RE, Park S, Perlin DS, et al. Multi-azole resistance in Aspergillus fumigatus. Int J Antimicrob Agents. 2006;28:450–3. https://doi.org/10.1016/j.ijantimicag.2006.08.017.
Hagiwara D, Arai T, Takahashi H, Kusuya Y, Watanabe A, Kamei K. Azole-Resistant Aspergillus fumigatus Isolates with mutation in HMG-CoA Reductase. Emerg Infect Dis. 2018;24:1889–97. https://doi.org/10.3201/eid2410.180730.
Perlin DS, Shor E, Zhao Y. Update on antifungal drug resistance. Curr Clin Microbiol Rep. 2015;2:84–95. https://doi.org/10.1007/s40588-015-0015-1.
Bellanger A-P, Berceanu A, Scherer E, Desbrosses Y, Daguindau E, Rocchi S, et al. Invasive fungal disease, isavuconazole treatment failure, and death in acute myeloid leukemia patients. Emerg Infect Dis. 2019;25:1778–9. https://doi.org/10.3201/eid2509.190598.
Pilmis B, Garcia-Hermoso D, Alanio A, Catherinot E, Scemla A, Jullien V, et al. Failure of voriconazole therapy due to acquired azole resistance in Aspergillus fumigatus in a kidney transplant recipient with chronic necrotizing aspergillosis. Am J Transplant. 2018;18:2352–5. https://doi.org/10.1111/ajt.14940.
Van Ingen J, van der Lee HAL, Rijs AJMM, Snelders E, Melchers WJG, Verweij PE. High-level pan-azole-resistant Aspergillosis: TABLE 1. Warnock DW, editor. J Clin Microbiol. 2015;53:2343–5. https://doi.org/10.1128/JCM.00502-15.
Lewis RE. Current concepts in antifungal pharmacology. Mayo Clin Proc. 2011 [cited 2019 Nov 12];86:805–817. https://linkinghub.elsevier.com/retrieve/pii/S0025619611651835
Andes D, Kovanda L, Desai A, Kitt T, Zhao M, Walsh TJ. Isavuconazole concentration in real-world practice: Consistency with results from clinical trials. Antimicrob Agents Chemother. 2018;62:e00585–18. https://doi.org/10.1128/AAC.00585-18.
Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: Pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother. 2017;72(1):i12–8. https://doi.org/10.1093/jac/dkx029.
Buil J, Hagen F, Chowdhary A, Verweij P, Meis J. Itraconazole, voriconazole, and posaconazole CLSI MIC distributions for wild-type and azole-resistant Aspergillus fumigatus isolates. J Fungi. 2018;4:103. https://doi.org/10.3390/jof4030103.
Lepak AJ, Marchillo K, VanHecker J, Andes DR. Posaconazole pharmacodynamic target determination against wild-type and cyp51 mutant isolates of aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2013;57:579–85. https://doi.org/10.1128/AAC.01279-12.
•• Schauwvlieghe AFAD, Buil JB, Verweij PE, Hoek RAS, Cornelissen JJ, Blijlevens NMA, et al. High-dose posaconazole for azole-resistant aspergillosis and other difficult-to-treat mould infections. Mycoses. 2019;myc.13028. https://doi.org/10.1111/myc.13028Recent clinical case series on the success of using high-dose posaconazole therapy for treatment of invasive fungal infections, including azole-resistant Aspergillus fumigatus.
Buil JB, Brüggemann RJM, Wasmann RE, Zoll J, Meis JF, Melchers WJG, et al. Isavuconazole susceptibility of clinical Aspergillus fumigatus isolates and feasibility of isavuconazole dose escalation to treat isolates with elevated MICs. J Antimicrob Chemother. 2018;73:134–42. https://doi.org/10.1093/jac/dkx354.
EUCAST. Isavuconazole and Aspergillus spp.; rationale for the clinical breakpoints. 2015;1-13. Retrieved from: http://www.eucast.org. Accessed November 12, 2019.
EUCAST. Antifungal clinical breakpoint table. 2018. Retrieved from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/Antifungal_breakpoints_v_9.0_180212.pdf. Accessed November 12, 2019.
Stevens VM, Mueller SW, Reynolds PM, et al. Extrapolating antifungal animal data to humans—Is it reliable? Curr Fungal Infect Rep. 2020. https://doi.org/10.1007/s12281-020-00370-x.
Seyedmousavi S, Melchers WJG, Mouton JW, Verweij PE. Pharmacodynamics and dose-response relationships of liposomal amphotericin B against different azole-resistant Aspergillus fumigatus isolates in a murine model of disseminated aspergillosis. Antimicrob Agents Chemother. 2013;57:1866–71. https://doi.org/10.1128/AAC.02226-12.
Seyedmousavi S, Mouton JW, Melchers WJG, Verweij PE. In vivo efficacy of liposomal amphotericin B against wild-type and azole-resistant aspergillus fumigatus isolates in two different immunosuppression models of invasive aspergillosis. Antimicrob Agents Chemother. 2017;61:e02479–16. https://doi.org/10.1128/AAC.02479-16.
Zoran T, Sartori B, Sappl L, Aigner M, Sánchez-Reus F, Rezusta A, et al. Azole-resistance in aspergillus terreus and related species: an emerging problem or a rare phenomenon? Front Microbiol. 2018;9:516. https://doi.org/10.3389/fmicb.2018.00516.
Viscoli C, Herbrecht R, Akan H, Baila L, Sonet A, Gallamini A, et al. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J Antimicrob Chemother. 2009;64:1274–81. https://doi.org/10.1093/jac/dkp355.
Herbrecht R, Maertens J, Baila L, Aoun M, Heinz W, Martino R, et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: An European Organisation for Research and Treatment of Cancer study. Bone Marrow Transplant. 2010;45:1227–33. https://doi.org/10.1038/bmt.2009.334.
Cornely OA, Vehreschild JJ, Vehreschild MJGT, Würthwein G, Arenz D, Schwartz S, et al. Phase II dose escalation study of caspofungin for invasive aspergillosis. Antimicrob Agents Chemother. 2011;55:5798–803. https://doi.org/10.1128/AAC.05134-11.
Jeans AR, Howard SJ, Al-Nakeeb Z, Goodwin J, Gregson L, Warn PA, et al. Combination of voriconazole and anidulafungin for treatment of triazole-resistant aspergillus fumigatus in an in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2012;56:5180–5. https://doi.org/10.1128/AAC.01111-12.
Seyedmousavi S, Bruggemann RJM, Melchers WJG, Rijs AJMM, Verweij PE, Mouton JW. Efficacy and pharmacodynamics of voriconazole combined with anidulafungin in azole-resistant invasive aspergillosis. J Antimicrob Chemother. 2013;68:385–93. https://doi.org/10.1093/jac/dks402.
Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann Intern Med. 2015;162:81. https://doi.org/10.7326/M13-2508.
Raffetin A, Courbin V, Jullien V, Dannaoui E. In vitro combination of isavuconazole with echinocandins against azole-susceptible and -resistant aspergillus spp. Antimicrob Agents Chemother. 2017;62:e01382–17. https://doi.org/10.1128/AAC.01382-17.
Rijnders B. PCR Based detection of azole resistance in A. fumigatus to improve patient outcome (AzorMan). https://clinicaltrials.gov/ct2/show/NCT03121235. .
Van der Linden JWM, Jansen RR, Bresters D, Visser CE, Geerlings SE, Kuijper EJ, et al. Azole-resistant central nervous system aspergillosis. Clin Infect Dis. 2009;48:1111–3. https://doi.org/10.1086/597465.
Verweij P, Dorsthorst TE, Janssen DT, Meis WH, Mouton JW. In vitro activities at pH 5.0 and pH 7.0 and in vivo efficacy of flucytosine against Aspergillus fumigatus. Antimicrob Agents Chemother. 2008;52:4483–5. https://doi.org/10.1128/AAC.00491-08.
Schmitt-Hoffmann A-H, Kato K, Townsend R, Potchoiba MJ, Hope WW, Andes D, et al. Tissue distribution and elimination of isavuconazole following single and repeat oral-dose administration of isavuconazonium sulfate to rats. Antimicrob Agents Chemother. 2017;61:e01292–17. https://doi.org/10.1128/AAC.01292-17.
Lamoth F, Mercier T, André P, Pagani JL, Pantet O, Maduri R, et al. Isavuconazole brain penetration in cerebral aspergillosis. J Antimicrob Chemother. 2019;74(6):1751–3. https://doi.org/10.1093/jac/dkz050.
Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–83. https://doi.org/10.1128/CMR.00007-10.
Rouzaud C, Jullien V, Herbrecht A, Palmier B, Lapusan S, Morgand M, et al. Isavuconazole diffusion in infected human brain. Antimicrob Agents Chemother. 2019;63:e02474–18. https://doi.org/10.1128/AAC.02474-18.
Funding
Study authors are supported by the Colorado Clinical and Translational Sciences Institute (CCTSI). The CCTSI is supported in part by Colorado CTSA Grant UL1TR001082 from NCATS/NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tyree Kiser reports grants from CTSA Grant Number UL1 TR002535 during the conduct of the study; and grants from Astellas, grants from Allergan and grants from Pfizer outside the submitted work. Scott Mueller reports grants from NCATS/NIH CTSA Grant Number UL1 TR002535 which supports the Colorado Clinical and Translational Sciences Institute; from CSL Behring Investigator-Initiated Study, grants from Merck Investigator-Initiated Studies Program; all industry funding has been received by the institution that employs Dr. Mueller, and is outside the submitted work (unrelated to antifungal therapeutics). Alison Novak and Mary Bradley declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pharmacology and Pharmacodynamics of Antifungal Agents
Rights and permissions
About this article
Cite this article
Novak, A.R., Bradley, M.E., Kiser, T.H. et al. Azole-Resistant Aspergillus and Echinocandin-Resistant Candida: What Are the Treatment Options?. Curr Fungal Infect Rep 14, 141–152 (2020). https://doi.org/10.1007/s12281-020-00379-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-020-00379-2