Abstract
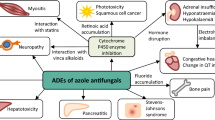
Various case reports have been published regarding the incidence of hepatotoxicity and the triazoles. To date, of the more commonly used triazoles, voriconazole has been liked to the highest incidence of transaminase elevations followed by posaconazole, fluconazole, and itraconazole, respectively. Discontinuation of each of the drugs has been shown to resolve the increase of transaminase levels; however, no clear guidance has been suggested on as to when discontinuation of therapy is warranted. Close monitoring particularly patients of Asian decent, underlying liver disease, bone marrow, or lung transplant may be prudent as well as target drug monitoring (TDM) for posaconazole and voriconazole to help assess the necessity of alteration or discontinuation of therapy.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
http://livertox.nlm.nih.gov/Fluconazole.htm Accessed 3/25/2015.
http://livertox.nih.gov/Itraconazole.htm Accessed 3/27/2015.
http://livertox.nlm.nih.gov/Posaconazole.htm Accessed 3/31/2015.
http://livertox.nlm.nih.gov/Voriconazole.htm Accessed 4/10/2015.
Diflucan® [package insert]. New York, NY. Pfizer. 2013.
Fischer MA, Winkelmayer WC, Rubin RH, Avorn J. The hepatotoxicity of antifungal medications in bone marrow transplant recipients. Clin Infect Dis. 2005;41:301–7.
Song J, Deresinski S. Hepatotoxicity of antifungal agents. Curr Opin Investig Drugs. 2005;6:170–7.
Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76.
Girois SB, Chapuis F, Decullier E, Revol BG. Adverse effects of antifungal therapies in invasive fungal infections: review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2006;25:138–49.
Wang JL, Chang CH, Young-Xu Y, Chan KA. Systematic review and meta-analysis of the tolerability and hepatotoxicity of antifungals in empirical and definitive therapy for invasive fungal infection. Antimicrob Agents Chemother. 2010;54:2409–19.
Moseley RH. Antifungal agents. Antibacterial and antifungal agents. In: Kaplowitz N, DeLeve LD, editors. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier; 2013. p. 470–81.
http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a4d555fa-787c-40fb-bb7d-b0d4f7318fd0 Accessed 3/27/2015
Somchit N, Norshahida AR, Hasiah AH, Zuraini A, et al. Hepatotoxicity induced by antifungal drugs itraconazole and fluconazole in rats: a comparative in vivo study. Hum Exp Toxicol. 2004;23:519–25.
Posaconazole. Clinical pharmacology; Gold Standard Multimedia. Accessed March 29, 2015
Raschi E, Poluzzi E, Koci A, Caraceni P, et al. Assessing liver injury associated with antimycotics: concise literature review and clues from data mining of the FAERS database. World J Hepatol. 2014;6(8):601–12. A compiled review from the FDA Adverse Events Reporting System showing that all triazole antifungals have been associated with clinical significant liver toxicity.
Foo H, Gottlieb T. Lack of cross-hepatotoxicity between voriconazole and posaconazole. Clin Infect Dis. 2007;45:803–5.
Pfizer Inc. Label: voriconazole for injection, tablets, oral suspension: LAB-0271-22.0; revised 2010 June.
Theuretzbacher U, Ihle F, Derendorf H. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin Pharmacokinet. 2006;45(7):649–63.
Levin MD, den Hollander JG, van der Holt B, et al. Hepatotoxicity of oral and intravenous voriconazole in relations to cytochrome P450 polymorphisms. J Antimicrob Chemother. 2007;60:1104–7.
den Hollander JG, van Arkel C, Rijnders BJ, et al. Incidence of voriconazole hepatotoxicity during intravenous and oral treatment for invasive fungal infections. J Antimicrob Chemother. 2006;57:1248–50.
Gorski E, Esterly JS, Postelnick M, et al. Evaluation of hepatotoxicity with off-label oral-treatment doses of voriconazole for invasive fungal infections. Antimicrob Agents Chemother. 2011;55(1):184–9.
Alffenaar JWC, van Assen S, de Monchy JGR, et al. Intravenous voriconazole after toxic oral administration. Antimicrob Agents Chemother. 2010;54(6):2741–2.
Luong ML, Hosseini-Moghaddam SM, Singer LG, et al. Risk factors for voriconazole hepatotoxicity at 12 weeks in lung transplant recipients. Am J Transplant. 2012;12(7):1929–35.
Belaiche S, Roustit M, Bedouch P, et al. Management of voriconazole hepatotoxicity in a lung transplant patient. Transpl Infect Dis. 2011;13:309–11.
Matsumoto K, Ikawa K, Abematsu K, et al. Correlation between voriconazole trough plasma concentration and hepatotoxicity in patients with different CYP2C19 genotypes. Int J Antimicrob Agents. 2009;34:91–4.
Hyland R, Jones BC, Smith DA. Identification of the cytochrome P450 enzymes involved in the N-oxidation of voriconazole. Drug Metab. 2003;31:540–7.
Wang T, Zhu H, Sun J, et al. Efficacy and safety of voriconazole and CYP2C19 polymorphism for optimised dosage regimens in patients with invasive fungal infections. Int J Antimicrob Agents. 2014;44:436–42. This study helped determine the optimum voriconazole target concentration and attempted to identify a dose-adjustment strategy for voriconazole according to CYP2C19 polymorphism.
Lutsar I, Hodges MR, Tomaszewski K, et al. Safety of voriconazole and dose individualization [letter]. Clin Infect Dis. 2003;36(8):1087–8.
Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–43.
Pascual A, Calandra T, Bolay S, et al. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis. 2008;46:201–11.
Mitsani D, Nguyen MH, Shields RK, et al. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob Agents Chemother. 2012;56(5):2371–7.
Chu HY, Jain R, Xie H, et al. Voriconazole therapeutic drug monitoring: retrospective cohort study of the relationship to clinical outcomes and adverse events. BMC Infect Dis. 2013;13:105.
Suzuki Y, Tokimatsu I, Sato Y, et al. Association of sustained high plasma trough concentration of voriconazole with the incidence of hepatotoxicity. Clin Chim Acta. 2013;424:119–22.
Dolton MJ, McLachlan AJ. Voriconazole pharmacokinetics and exposure–response relationships: assessing the links between exposure, efficacy and toxicity. Int J Antimicrob Agents. 2014;44:183–93. Excellent review providing a critical analysis on voriconazole pharmacokinetics, exposure response relationships and toxicity.
Compliance with Ethics Guidelines
Conflict of Interest
Viktorija O. Barr, Elizabeth G. Zdyb, and Michael Postelnick declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Pharmacology and Pharmacodynamics of Antifungal Agents
Rights and permissions
About this article
Cite this article
Barr, V.O., Zdyb, E.G. & Postelnick, M. The Clinical Significance of Azole Antifungals’ Effects on the Liver and Transaminase Levels. Curr Fungal Infect Rep 9, 190–195 (2015). https://doi.org/10.1007/s12281-015-0226-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12281-015-0226-1