Abstract
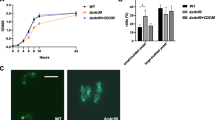
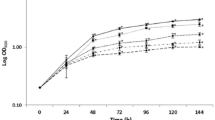
Aspergillus fumigatus is a well-known opportunistic pathogen that causes invasive aspergillosis (IA) infections with high mortality in immunosuppressed individuals. Morphogenesis, including hyphal growth, conidiation, and cell wall biosynthesis is crucial in A. fumigatus pathogenesis. Based on a previous random insertional mutagenesis library, we identified the putative polysaccharide synthase gene Afcps1 and its para-log Afcps2. Homologs of the cps gene are commonly found in the genomes of most fungal and some bacterial pathogens. Afcps1/cpsA is important in sporulation, cell wall composition, and virulence. However, the precise regulation patterns of cell wall integrity by Afcps1/cpsA and further effects on the immune response are poorly understood. Specifically, our in-depth study revealed that Afcps1 affects cell-wall stability, showing an increased resistance of ΔAfcps1 to the chitinmicrofibril destabilizing compound calcofluor white (CFW) and susceptibility of ΔAfcps1 to the β-(1,3)-glucan synthase inhibitor echinocandin caspofungin (CS). Additionally, deletion of Afcps2 had a normal sporulation phenotype but caused hypersensitivity to Na+ stress, CFW, and Congo red (CR). Specifically, quantitative analysis of cell wall composition using high-performance anion exchange chromatography-pulsed amperometric detector (HPAEC-PAD) analysis revealed that depletion of Afcps1 reduced cell wall glucan and chitin contents, which was consistent with the down-regulation of expression of the corresponding biosynthesis genes. Moreover, an elevated immune response stimulated by conidia of the ΔAfcps1 mutant in marrow-derived macrophages (BMMs) during phagocytosis was observed. Thus, our study provided new insights into the function of polysaccharide synthase Cps1, which is necessary for the maintenance of cell wall stability and the adaptation of conidia to the immune response of macrophages in A. fumigatus.
Similar content being viewed by others
References
Akoumianaki, T., Kyrmizi, I., Valsecchi, I., Gresnigt, M.S., Samonis, G., Drakos, E., Boumpas, D., Muszkieta, L., Prevost, M.C., Kontoyiannis, D.P., et al. 2016. Aspergillus cell wall melanin blocks LC3-associated phagocytosis to promote pathogenicity. Cell Host Microbe 19, 79–90.
Beauvais, A., Maubon, D., Park, S., Morelle, W., Tanguy, M., Huerre, M., Perlin, D.S., and Latgé, J.P. 2005. Two a(1–3) glucan synthases with different functions in Aspergillus fumigatus. Appl. Environ. Microbiol. 71, 1531–1538.
Bleichrodt, R.J., Foster, P., Howell, G., Latgé, J.P., and Read, N.D. 2020. Cell wall composition heterogeneity between single cells in Aspergillus fumigatus leads to heterogeneous behavior during antifungal treatment and phagocytosis. mBio 11, e03015–19.
Caffrey, A.K., Lehmann, M.M., Zickovich, J.M., Espinosa, V., Shepardson, K.M., Watschke, C.P., Hilmer, K.M., Thammahong, A., Barker, B.M., Rivera, A., et al. 2015. IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS Pathog. 11, e1004625.
Cartee, R.T., Forsee, W.T., Schutzbach, J.S., and Yother, J. 2000. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J. Biol. Chem. 275, 3907–3914.
Chang, Y.C., Jong, A., Huang, S., Zerfas, P., and Kwon-Chung, K.J. 2006. CPS1, a homolog of the Streptococcus pneumoniae type 3 polysaccharide synthase gene, is important for the pathobiology of Cryptococcus neoformans. Infect. Immun. 74, 3930–3938.
Combier, J.P., Melayah, D., Raffier, C., Gay, G., and Marmeisse, R. 2003. Agrobacterium tumefaciens-mediated transformation as a tool for insertional mutagenesis in the symbiotic ectomycorrhizal fungus Hebeloma cylindrosporum. FEMS Microbiol. Lett. 220, 141–148.
Cortés, J.C.G., Curto, M.Á., Carvalho, V.S.D., Pérez, P., and Ribas, J.C. 2019. The fungal cell wall as a target for the development of new antifungal therapies. Biotechnol. Adv. 37, 107352.
Dagenais, T.R. and Keller, N.P. 2009. Pathogenesis of Aspergillus fumigatus in invasive Aspergillosis. Clin. Microbiol. Rev. 22, 447–465.
Dichtl, K., Samantaray, S., Aimanianda, V., Zhu, Z., Prévost, M.C., Latgé, J.P., Ebel, F., and Wagener, J. 2015. Aspergillus fumigatus devoid of cell wall β-1,3-glucan is viable, massively sheds galactomannan and is killed by septum formation inhibitors. Mol. Microbiol. 95, 458–471.
Dillard, J.P., Vandersea, M.W., and Yother, J. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181, 973–983.
Erjavec, Z., Kluin-Nelemans, H., and Verweij, P.E. 2009. Trends in invasive fungal infections, with emphasis on invasive aspergillosis. Clin. Microbiol. Infect. 15, 625–633.
Fang, W., Sanz, A.B., Bartual, S.G., Wang, B., Ferenbach, A.T., ParkaŠ, V., Hurtado-Guerrero, R., Arroyo, J., and van Aalten, D.M.F. 2019. Mechanisms of redundancy and specificity of the Aspergillus fumigatus Crh transglycosylases. Nat. Commun. 10, 1669.
Feng, X.H., Ramamoorthy, V., Pandit, S.S., Prieto, A., Espeso, E.A., and Calvo, A.M. 2017. cpsA regulates mycotoxin production, morphogenesis and cell wall biosynthesis in the fungus Aspergillus nidulans. Mol. Microbiol. 105, 1–24.
Fontaine, T., Beauvais, A., Loussert, C., Thevenard, B., Fulgsang, C.C., Ohno, N., Clavaud, C., Prevost, M.C., and Latgé, J.P. 2010. Cell wall α1–3glucans induce the aggregation of germinating conidia of Aspergillus fumigatus. Fungal Genet. Biol. 47, 707–712.
Fontaine, T., Simenel, C., Dubreucq, G., Adam, O., Delepierre, M., Lemoine, J., Vorgias, C.E., Diaquin, M., and Latgé, J.P. 2000. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J. Biol. Chem. 275, 27594–27607.
Fortwendel, J.R., Juvvadi, P.R., Perfect, B.Z., Rogg, L.E., Perfect, J.R., and Steinbach, W.J. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob. Agents Chemother. 54, 1555–1563.
Fortwendel, J.R., Juvvadi, P.R., Pinchai, N., Perfect, B.Z., Alspaugh, J.A., Perfect, J.R., and Steinbach, W.J. 2009. Differential effects of inhibiting chitin and 1,3-β-D-glucan synthesis in Ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53, 476–482.
Fu, C., Sokolow, E., Rupert, C.B., and Free, S.J. 2014. The Neurospora crassa CPS-1 polysaccharide synthase functions in cell wall biosynthesis. Fungal Genet. Biol. 69, 23–30.
Gastebois, A., Clavaud, C., Aimanianda, V., and Latgé, J.P. 2009. Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiol. 4, 583–595.
Geißel, B., Loiko, V., Klugherz, I., Zhu, Z., Wagener, N., Kurzai, O., van den Hondel, C.A., and Wagener, J. 2018. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 9, 3098.
Ghasemi, H., Ghazanfari, T., Yaraee, R., Owlia, P., Hassan, Z.M., and Faghihzadeh, S. 2012. Roles of IL-10 in ocular inflammations: a review. Ocul. Immunol. Inflamm. 20, 406–418.
Gravelat, F.N., Beauvais, A., Liu, H., Lee, M.J., Snarr, B.D., Chen, D., Xu, W., Kravtsov, I., Hoareau, C.M., Vanier, G., et al. 2013. Aspergillus galactosaminogalactan mediates adherence to host constituents and conceals hyphal β-glucan from the immune system. PLoS Pathog. 9, e1003575.
Hasim, S. and Coleman, J.J. 2019. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med. Chem. 11, 869–883.
Henry, C., Latgé, J.P., and Beauvais, A. 2012. α1,3 glucans are dispensable in Aspergillus fumigatus. Eukaryot. Cell 11, 26–29.
Hohl, T.M., Feldmesser, M., Perlin, D.S., and Pamer, E.G. 2008. Caspofungin modulates inflammatory responses to Aspergillus fumigatus through stage-specific effects on fungal β-glucan exposure. J. Infect. Dis. 198, 176–185.
Hopke, A., Brown, A.J.P., Hall, R.A., and Wheeler, R.T. 2018. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 26, 284–295.
Ibrahim-Granet, O., Philippe, B., Boleti, H., Boisvieux-Ulrich, E., Grenet, D., Stern, M., and Latgé, J.P. 2003. Phagocytosis and intracellular fate of Aspergillus fumigatus conidia in alveolar macrophages. Infect. Immun. 71, 891–903.
Jong, A., Wu, C.H., Chen, H.M., Luo, F., Kwon-Chung, K.J., Chang, Y.C., LaMunyon, C.W., Plaas, A., and Huang, S.H. 2007. Identification and characterization of CPS1 as a hyaluronic acid synthase contributing to the pathogenesis of Cryptococcus neoformans infection. Eukaryot. Cell 6, 1486–1496.
Kędzierska, A., Kochan, P., Pietrzyk, A., and Kedzierska, J. 2007. Current status of fungal cell wall components in the immunodiagnostics of invasive fungal infections in humans: galactomannan, mannan and (1→3)-β-D-glucan antigens. Eur. J. Clin. Microbiol. Infect. Dis. 26, 755–766.
Kopecká, M. and Gabriel, M. 1992. The influence of Congo red on the cell wall and (1→3)-β-D-glucan microfibril biogenesis in Saccharomyces cerevisiae. Arch. Microbiol. 158, 115–126.
Kyrmizi, I., Gresnigt, M.S., Akoumianaki, T., Samonis, G., Sidiropoulos, P., Boumpas, D., Netea, M.G., van de Veerdonk, F.L., Kontoyiannis, D.P., and Chamilos, G. 2013. Corticosteroids block autophagy protein recruitment in Aspergillus fumigatus phagosomes via targeting dectin-1/Syk kinase signaling. J. Immunol. 191, 1287–1299.
Latgé, J.P. 2007. The cell wall: a carbohydrate armour for the fungal cell. Mol. Microbiol. 66, 279–290.
Latgé, J.P. 2010. Tasting the fungal cell wall. Cell. Microbiol. 12, 863–872.
Latgé, J.P., Beauvais, A., and Chamilos, G. 2017. The cell wall of the human fungal pathogen Aspergillus fumigatus: biosynthesis, organization, immune response, and virulence. Annu. Rev. Microbiol. 71, 99–116.
Lee, M.J. and Sheppard, D.C. 2016. Recent advances in the understanding of the Aspergillus fumigatus cell wall. J. Microbiol. 54, 232–242.
Long, N., Zeng, L., Qiao, S., Li, L., and Zhong, G. 2018. Aspergillus fumigatus Afssn3-Afssn8 pair reverse regulates azole resistance by conferring extracellular polysaccharide, sphingolipid pathway intermediates, and efflux pumps to biofilm. Antimicrob. Agents Chmother. 62, e01978–17.
Maubon, D., Park, S., Tanguy, M., Huerre, M., Schmitt, C., Prévost, M.C., Perlin, D.S., Latgé, J.P., and Beauvais, A. 2006. AGS3, an α(1–3)glucan synthase gene family member of Aspergillus fumigatus, modulates mycelium growth in the lung of experimentally infected mice. Fungal Genet. Biol. 43, 366–375.
Mellado, E., Dubreucq, G., Mol, P., Sarfati, J., Paris, S., Diaquin, M., Holden, D.W., Rodriguez-Tudela, J.L., and Latgé, J.P. 2003. Cell wall biogenesis in a double chitin synthase mutant (chsG−/chsE−) of Aspergillus fumigatus. Fungal Genet. Biol. 38, 98–109.
Mennink-Kersten, M.A., Donnelly, J.P., and Verweij, P.E. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4, 349–357.
Mouyna, I., Hartland, R.P., Fontaine, T., Diaquin, M., Simenel, C., Delepierre, M., Henrissat, B., and Latgé, J.P. 1998. A 1,3-β-glucanosyltransferase isolated from the cell wall of Aspergillus fumigatus is a homologue of the yeast Bgl2p. Microbiology 144, 3171–3180.
Nepal, B., Myers, R., Lohmar, J.M., Puel, O., Thompson, B., Van Cura, M., and Calvo, A.M. 2019. Characterization of the putative polysaccharide synthase CpsA and its effects on the virulence of the human pathogen Aspergillus fumigatus. PLoS ONE 14, e0216092.
Park, H.S., Yu, M.Y., Lee, M.K., Maeng, P.J., Kim, S.C., and Yu, J.H. 2015. Velvet-mediated repression of β-glucan synthesis in Aspergillus nidulans spores. Sci. Rep. 5, 10199.
Ram, A.F.J. and Klis, F.M. 2006. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat. Protoc. 1, 2253–2256.
Romani, L. 2011. Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288.
Silva, R.L., Lopes, A.H., Guimarães, R.M., and Cunha, T.M. 2017. CXCL1/CXCR2 signaling in pathological pain: role in peripheral and central sensitization. Neurobiol. Dis. 105, 109–116.
Sugui, J.A., Chang, Y.C., and Kwon-Chung, K.J. 2005. Agrobacterium tumefaciens-mediated transformation of Aspergillus fumigatus: an efficient tool for insertional mutagenesis and targeted gene disruption. Appl. Environ. Microbiol. 71, 1798–1802.
Turner, M.S., Drew, R.H., and Perfect, J.R. 2006. Emerging echinocandins for treatment of invasive fungal infections. Expert Opin. Emerg. Drugs 11, 231–250.
Vessels, J.M. and Radding, J.A. 1993. Oligosaccharide mapping of fungal glucan synthase product by high-performance anion-exchange chromatography. Anal. Biochem. 215, 150–155.
Yoshimi, A., Sano, M., Inaba, A., Kokubun, Y., Fujioka, T., Mizutani, O., Hagiwara, D., Fujikawa, T., Nishimura, M., Yano, S., et al. 2013. Functional analysis of the α-1,3-glucan synthase genes agsA and agsB in Aspergillus nidulans: AgsB is the major α-1,3-glucan synthase in this fungus. PLoS ONE 8, e54893.
Zhang, Z., Khan, N.M., Nunez, K.M., Chess, E.K., and Szabo, C.M. 2012. Complete monosaccharide analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Chem. 84, 4104–4110.
Acknowledgments
This work was financially supported by the Natural Science Foundation of Zhejiang (LY19C010001), the National Natural Science Foundation of China (NSFC) (31500055 and 31901668), Scientific Research Fund of Zhejiang Provincial Education Department (Y201940932), the Natural Science Foundation of Ningbo (2019A610436), and School Research Project in Ningbo University (XYL19011). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest
The authors report no conflict of interest.
Financial Disclosure
The authors report no financial interest or benefit arising from the direct application of this work.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Wang, S., Yuan, A., Zeng, L. et al. The putative polysaccharide synthase AfCps1 regulates Aspergillus fumigatus morphogenesis and conidia immune response in mouse bone marrow-derived macrophages. J Microbiol. 59, 64–75 (2021). https://doi.org/10.1007/s12275-021-0347-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-021-0347-x