Abstract
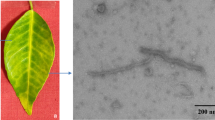
Trichoderma atroviride is a common fungus found in various ecosystems that shows mycoparasitic ability on other fungi. A novel dsRNA virus was isolated from T. atroviride NFCF377 strain and its molecular features were analyzed. The viral genome consists of a single segmented double-stranded RNA and is 9,584 bp in length, with two discontinuous open reading frames (ORF1 and ORF2). A mycoviral structural protein and an RNA-dependent RNA polymerase (RdRp) are encoded by ORF1 and ORF2, respectively, between which is found a canonical shifty heptameric signal motif (AAAAAAC) followed by an RNA pseudoknot. Analysis of sequence similarity and phylogeny showed that it is closely related to members of the proposed family “Fusagraviridae”, with a highest similarity to the Trichoderma atroviride mycovirus 1 (TaMV1). Although the sequence similarity of deduced amino acid to TaMV1 was evident, sequence deviations were distinctive at untranslated regions (UTRs) due to the extended size. Thus, we inferred this dsRNA to be a different strain of Trichoderma atroviride mycovirus 1 (TaMV1-NFCF377). Electron microscopy image exhibited an icosahedral viral particle of 40 nm diameter. Virus-cured isogenic isolates were generated and no differences in growth rate, colony morphology, or conidia production were observed between virus-infected and virus-cured strains. However, culture filtrates of TaMV1-NFCF377-infected strain showed enhanced antifungal activity against the plant pathogen Rhizoctonia solani but not to edible mushroom Pleurotus ostreatus. These results suggested that TaMV1-NFCF377 affected the metabolism of the fungal host to potentiate antifungal compounds against a plant pahogen, but this enhanced antifungal activity appeared to be species-specific.
Similar content being viewed by others
References
Ahn, I.P. and Lee, Y.H. 2001. A viral double-stranded RNA up regulates the fungal virulence of Nectria radicicola. Mol. Plant Microbe Interact. 14, 496–507.
Andika, I.B., Wei, S., Cao, C., Salaipeth, L., Kondo, H., and Sun, L. 2017. Phytopathogenic fungus hosts a plant virus: A naturally occurring cross-kingdom viral infection. Proc. Natl. Acad. Sci. USA 114, 12267–12272.
Anfoka, C. and Buchenauer, H. 1997. Systemic acquired resistance in tomato against Phytophthora infestans by pre-inoculation with tobacco necrosis virus. Physiol. Mol. Plant Pathol. 50, 85–101.
Arjona-Lopez, J.M., Telengech, P., Jamal, A., Hisano, S., Kondo, H., Yelin, M.D., Arjona-Girona, I., Kanematsu, S., Lopez-Herrera, C.J., and Suzuki, N. 2018. Novel, diverse RNA viruses from Mediterranean isolates of the phytopathogenic fungus, Rosellinia necatrix: insights into evolutionary biology of fungal viruses. Environ. Microbiol. 20, 1464–1483.
Asad, S.A., Ali, N., Hameed, A., Khan, S.A., Ahmad, R., Bilal, M., Shahzad, M., and Tabassum, A. 2014. Biocontrol efficacy of different isolates of Trichoderma against soil borne pathogen Rhizoctonia solani. Pol. J. Microbiol. 63, 95–103.
Bidet, K. and Garcia-Blanco, M.A. 2014. Flaviviral RNAs: weapons and targets in the war between virus and host. Biochem. J. 462, 215–230.
Brierley, I. 1995. Ribosomal frameshifting on viral RNAs. J. Gen. Virol. 76, 1885–1892.
Brierley, I., Pennell, S., and Gilbert, R.J.C. 2007. Viral RNA pseudoknots: versatile motifs in gene expression and replication. Nat. Rev. Microbiol. 5, 598–610.
Chun, J., Yang, H.E., and Kim, D.H. 2018a. Identification and molecular characterization of a novel partitivirus from Trichoderma atroviride NFCF394. Viruses 10, 578.
Chun, J., Yang, H.E., and Kim, D.H. 2018b. Identification of a novel partitivirus of Trichoderma harzianum NFCF319 and evidence for the related antifungal activity. Front. Plant Sci. 9, 1699.
Coninck, E., Scauflaire, J., Gollier, M., Liénard, C., Foucart, G., Manssens, G., Munaut, F., and Legrève, A. 2020. Trichoderma atroviride as a promising biocontrol agent in seed coating for reducing Fusarium damping-off on maize. J. Appl. Microbiol. 129, 637–651.
Dennis, C. and Webster, J. 1971. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 57, 41–48.
de Sá, P.B., Havens, W.M., and Ghabrial, S.A. 2010. Characterization of a novel broad-spectrum antifungal protein from virus-infected Helminthosporium (Cochliobolus) victoriae. Phytopathology 100, 880–889.
Eusebio-Cope, A., Sun, L., Tanaka, T., Chiba, S., Kasahara, S., and Suzuki, N. 2015. The chestnut blight fungus for studies on virus/host and virus/virus interactions: from a natural to a model host. Virology 477, 164–175.
Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791.
Gebhard, L.G., Filomatori, C.V., and Gamarnik, A.V. 2011. Functional RNA elements in the dengue virus genome. Viruses 3, 1739–1756.
Ghabrial, S.A., Castón, J.R., Jiang, D., Nibert, M.L., and Suzuki, N. 2015. 50-plus years of fungal viruses. Virology 479–480, 356–368.
Ghabrial, S.A. and Suzuki, N. 2009. Viruses of plant pathogenic fungi. Annu. Rev. Phytopathol. 47, 353–384.
Gomes, R.C., Sêmedo, L.T.A.S., Soares, R.M.A., Linhares, L.F., Ulhoa, C.J., Alviano, C.S., and Coelho, R.R. 2001. Purification of a thermostable endochitinase from Streptomyces RC1071 isolated from a cerrado soil and its antagonism against phytopathogenic fungi. J. Appl. Microbiol. 90, 653–661.
Kanhayuwa, L., Kotta-Loizou, I., Özkan, S., Gunning, A.P., and Coutts, R.H.A. 2015. A novel mycovirus from Aspergillus fumigatus contains four unique dsRNAs as its genome and is infectious as dsRNA. Proc. Natl. Acad. Sci. USA 112, 9100–9105.
Kim, J.M., Jung, J.E., Park, J.A., Park, S.M., Cha, B.J., and Kim, D.H. 2013. Biological function of a novel chrysovirus, CnV1-BS122, in the Korean Cryphonectria nitschkei BS122 strain. J. Biosci. Bioeng. 115, 1–3.
Kombrink, E. and Somssich, I.E. 1997. Pathogenesis-related proteins and plant defense. In Carroll, G.C. and Tudzynski, P. (eds.), The Mycota: A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research, vol. 5, pp. 107–128. Springer, Berlin, Germany.
Kotta-Loizou, I. and Coutts, R.H.A. 2017. Studies on the virome of the entomopathogenic fungus Beauveria bassiana reveal novel dsRNA elements and mild hypervirulence. PLoS Pathog. 13, e1006183.
Kumar, S., Stecher, G., and Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874.
Lee, S.H., Yun, S.H., Chun, J., and Kim, D.H. 2017. Characterization of a novel dsRNA mycovirus of Trichoderma atroviride NFCF028. Arch. Virol. 162, 1073–1077.
Meena, M., Swapnil, P., Zehra, A., Dubey, M.K., and Upadhyay, R.S. 2017. Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch. Phytopathol. Plant Prot. 50, 629–648.
Nakamura, H., Ikeda, K., Arakawa, M., and Matsumoto, N. 2002. Conidioma production of the white root rot fungus in axenic culture under near-ultraviolet light radiation. Mycoscience 43, 251–254.
Nawrocka, J., Gromek, A., and Malolepsza, U. 2019. Nitric oxide as a beneficial signaling molecule in Trichoderma atroviride TRS25-induced systemic defense responses of cucumber plants against Rhizoctonia solani. Front. Plant Sci. 10, 421.
Nuss, D.L. 2005. Hypovirulence: mycoviruses at the fungal-plant interface. Nat. Rev. Microbiol. 3, 632–642.
Park, S.M., Choi, E.S., Kim, M.J., Cha, B.J., Yang, M.S., and Kim, D.H. 2004. Characterization of HOG1 homologue, CpMK1, from Cryphonectria parasitica and evidence for hypovirus-mediated perturbation of its phosphorylation in response to hypertonic stress. Mol. Microbiol. 51, 1267–1277.
Park, S.M., Kim, J.M., Chung, H.J., Lim, J.Y., Kwon, B.R., Lim, J.G., Kim, J.A., Kim, M.J., Cha, B.J., Lee, S.H., et al. 2008. Occurrence of diverse dsRNA in a Korean population of the chestnut blight fungus, Cryphonectria parasitica. Mycol. Res. 112, 1220–1226.
Polacheck, I. and Rosenberger, R.F. 1978. Distribution of autolysins in hyphae of Aspergillus nidulans: evidence for a lipid-mediated attachment to hyphal walls. J. Bacteriol. 135, 741–747.
Seaby, D. 1998. Trichoderma as a weed mould or pathogen in mushroom cultivation. In Harman, G.E. and Kubicek, C.P. (eds.), Trichoderma and Gliocladium. vol. 2, pp. 267–288. Taylor & Francis, London, the United Kingdom.
Stoppacher, N., Kluger, B., Zeilinger, S., Krska, R., and Schuhmacher, R. 2010. Identification and profiling of volatile metabolites of the biocontrol fungus Trichoderma atroviride by HS-SPME-GC-MS. J. Microbiol. Methods 81, 187–193.
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882.
Wang, L., Jiang, J., Wang, Y., Hong, N., Zhang, F., Xu, W., and Wang, G. 2014. Hypovirulence of the phytopathogenic fungus Botryosphaeria dothidea: association with a coinfecting chrysovirus and a partitivirus. J. Virol. 88, 7517–7527.
Wang, L., Zhang, J., Zhang, H., Qiu, D., and Guo, L. 2016. Two novel relative double-stranded RNA mycoviruses infecting Fusarium poae strain SX63. Int. J. Mol. Sci. 17, 641.
Weiler, F., Rehfeldt, K., Bautz, F., and Schmitt, M.J. 2002. The Zygosaccharomyces bailii antifungal virus toxin zygocin: cloning and expression in a heterologous fungal host. Mol. Microbiol. 46, 1095–1105.
Wheatley, R., Hackett, C., Bruce, A., and Kundzewicz, A. 1997. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. inhibitory to wood decay fungi. Int. Biodeterior. Biodegradation 39, 199–205.
Wickner, R.B. 1992. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu. Rev. Microbiol. 46, 347–375.
Wu, M.D., Jin, F.Y., Zhang, J., Yang, L., Jiang, D., and Li, G. 2012. Characterization of a novel bipartite double-stranded RNA mycovirus conferring hypovirulence in the phytopathogenic fungus Botrytis porri. J. Virol. 86, 6605–6619.
Yun, S.H., Lee, S.H., So, K.K., Kim, J.M., and Kim, D.H. 2016. Incidence of diverse dsRNA mycoviruses in Trichoderma spp. causing green mold disease of shiitake Lentinula edodes. FEMS Microbiol. Lett. 363, fnw220.
Acknowledgments
We wish to thank the Institute of Molecular Biology and Genetics at Jeonbuk National University for kindly providing the facilities for this research. This work was supported by the NRF grants from NRF-2018R1A2A1A05078682 and NRF-2019R1I1A1A01061618. This research was supported by “Research Base Construction Fund Support Program” funded by Jeonbuk National University in 2020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
Authors declare no competing interests.
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Chun, J., Na, B. & Kim, DH. Characterization of a novel dsRNA mycovirus of Trichoderma atroviride NFCF377 reveals a member of “Fusagraviridae” with changes in antifungal activity of the host fungus. J Microbiol. 58, 1046–1053 (2020). https://doi.org/10.1007/s12275-020-0380-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-020-0380-1