Abstract
Pulmonary tuberculosis (TB) is caused by Mycobacterium tuberculosis. The protein composition of sputum may reflect the immune status of the lung. This study aimed to evaluate the protein profiles in spontaneous sputum samples from patients with active pulmonary TB. Sputum samples were collected from patients with pulmonary TB and healthy controls. Western blotting was used to analyze the amount of interleukin 10 (IL-10), interferon-gamma (IFN-γ), IL-25, IL-17, perforin-1, urease, albumin, transferrin, lactoferrin, adenosine deaminase (also known as adenosine aminohydrolase, or ADA), ADA-2, granzyme B, granulysin, and caspase-1 in sputum. Results of detection of IL-10, IFN-γ, perforin-1, urease, ADA2, and caspase-1, showed relatively high specificity in distinguishing patients with TB from healthy controls, although sensitivities varied from 13.3% to 66.1%. By defining a positive result as the detection of any two proteins in sputum samples, combined use of transferrin and urease as markers increased sensitivity to 73.2% and specificity to 71.1%. Furthermore, we observed that the concentration of transferrin was proportional to the number of acid-fast bacilli detected in sputum specimens. Detection of sputum transferrin and urease was highly associated with pulmonary TB infection. In addition, a high concentration of transferrin detected in sputum might correlate with active TB infection. This data on sputum proteins in patients with TB may aid in the development of biomarkers to assess the severity of pulmonary TB.
Similar content being viewed by others
References
Boelaert, J.R., Vandecasteele, S.J., Appelberg, R., and Gordeuk, V.R. 2007. The effect of the host’s iron status on tuberculosis. J. Infect. Dis. 195, 1745–1753.
Boradia, V.M., Malhotra, H., Thakkar, J.S., Tillu, V.A., Vuppala, B., Patil, P., Sheokand, N., Sharma, P., Chauhan, A.S., Raje, M., et al. 2014. Mycobacterium tuberculosis acquires iron by cell-surface sequestration and internalization of human holo-transferrin. Nat. Commun. 5, 4730
Chou, C.H., Huang, Y.T., Hsu, H.L., Lai, C.C., Liao, C.H., and Hsueh, P.R. 2009. Rapid identification of the Mycobacterium tuberculosis complex by an enzyme-linked immunosorbent assay. Int. J. Tuberc. Lung Dis. 13, 996–1001.
Cooper, A.M., Dalton, D.K., Stewart, T.A., Griffin, J.P., Russell, D.G., and Orme, I.M. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178, 2243–2247.
Dilmac, A., Ucoluk, G.O., Ugurman, F., Gozu, A., Akkalyoncu, B., Eryilmaz, T., and Samurkasoglu, B. 2002. The diagnostic value of adenosine deaminase activity in sputum in pulmonary tuberculosis. Respir. Med. 96, 632–634.
Dimakou, K., Hillas, G., and Bakakos, P. 2009. Adenosine deaminase activity and its isoenzymes in the sputum of patients with pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 13, 744–748.
Forbes, B.A., Sahm, D.F., and Weissfeld, A.S. 2007. Bailey and Scott’s diagnostic microbiology. In Forbes, B.A., Sahm, D.F., and Weissfeld, A.S. (eds.) 12th ed, pp. 478–509. St. Louis, USA.
Fu, Y.R., Yi, Z.J., Guan, S.Z., Zhang, S.Y., and Li, M. 2012. Proteomic analysis of sputum in patients with active pulmonary tuberculosis. Clin. Microbiol. Infect. 18, 1241–1247.
Golub, J.E., Bur, S., Cronin, W.A., Gange, S., Baruch, N., Comstock, G.W., and Chaisson, R.E. 2006. Delayed tuberculosis diagnosis and tuberculosis transmission. Int. J. Tuberc. Lung Dis. 10, 24–30.
Horng, Y.T., Jeng, W.Y., Chen, Y.Y., Liu, C.H., Dou, H.Y., Lee, J.J., Chang, K.C., Chien, C.C., and Soo, P.C. 2015. Molecular analysis of codon 548 in the rpoB gene involved in Mycobacterium tuberculosis resistance to rifampin. Antimicrob. Agents Chemother. 59, 1542–1548.
John, S.H., Kenneth, J., and Gandhe, A.S. 2012. Host biomarkers of clinical relevance in tuberculosis: review of gene and protein expression studies. Biomarkers 17, 1–8.
Konieczna, I., Zarnowiec, P., Kwinkowski, M., Kolesinska, B., Fraczyk, J., Kaminski, Z., and Kaca, W. 2012. Bacterial urease and its role in long-lasting human diseases. Curr. Protein Pept. Sci. 13, 789–806.
Küpeli, E., Karnak, D., Beder, S., Kayacan, O., and Tutkak, H. 2008. Diagnostic accuracy of cytokine levels (TNF-alpha, IL-2, and IFNgamma) in bronchoalveolar lavage fluid of smear-negative pulmonary tuberculosis patients. Respiration 75, 73–78.
Lago, P.M., Boechat, N., Migueis, D.P., Almeida, A.S., Lazzarini, L.C., Saldanha, M.M., Kritski, A.L., Ho, J.L., and Lapa e Silva, J.R. 2012. Interleukin-10 and interferon-gamma patterns during tuberculosis treatment: possible association with recurrence. Int. J. Tuberc. Lung Dis. 16, 656–659.
Li, Q., Li, J., Tian, J., Zhu, B., Zhang, Y., Yang, K., Ling, Y., and Hu, Y. 2012. IL-17 and IFN-gamma production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. Eur. Rev. Med. Pharmacol. Sci. 16, 2029–2036.
Mindolli, P.B., Salmani, M.P., and Parandekar, P.K. 2013. Improved diagnosis of pulmonary tuberculosis using bleach microscopy method. J. Clin. Diagn. Res. 7, 1336–1338.
Moon, H.W. and Hur, M. 2013. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection: an updated review. Ann. Clin. Lab. Sci. 43, 221–229.
Murray, P.R. and Washington, J.A. 1975. Microscopic and baceriologic analysis of expectorated sputum. Mayo Clin. Proc. 50, 339–344.
Pitabut, N., Sakurada, S., Tanaka, T., Ridruechai, C., Tanuma, J., Aoki, T., Kantipong, P., Piyaworawong, S., Kobayashi, N., Dhepakson, P., et al. 2013. Potential function of granulysin, other related effector molecules and lymphocyte subsets in patients with TB and HIV/TB coinfection. Int. J. Med. Sci. 10, 1003–1014.
Ribeiro-Rodrigues, R., Resende Co, T., Johnson, J.L., Ribeiro, F., Palaci, M., Sa, R.T., Maciel, E.L., Pereira Lima, F.E., Dettoni, V., Toossi, Z., et al. 2002. Sputum cytokine levels in patients with pulmonary tuberculosis as early markers of mycobacterial clearance. Clin. Diagn. Lab. Immunol. 9, 818–823.
Sambrook, J., Fritsch, E.F., and Maniatis, T. 1989. Molecular cloning: a laboratory manual, 2nd ed, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA.
Sia, I.G. and Wieland, M.L. 2011. Current concepts in the management of tuberculosis. Mayo Clin. Proc. 86, 348–361.
Soo, P.C., Horng, Y.T., Chang, K.C., Wang, J.Y., Hsueh, P.R., Chuang, C.Y., Lu, C.C., and Lai, H.C. 2009. A simple gold nanoparticle probes assay for identification of Mycobacterium tuberculosis and Mycobacterium tuberculosis complex from clinical specimens. Mol. Cell. Probes 23, 240–246.
Soo, P.C., Horng, Y.T., Chen, A.T., Yang, S.C., Chang, K.C., Lee, J.J., and Peng, W.P. 2015. Validation of nanodiamond-extracted CFP- 10 antigen as a biomarker in clinical isolates of Mycobacterium tuberculosis complex in broth culture media. Tuberculosis 95, 620–624.
Soo, P.C., Horng, Y.T., Hsueh, P.R., Shen, B.J., Wang, J.Y., Tu, H.H., Wei, J.R., Hsieh, S.C., Huang, C.C., and Lai, H.C. 2006. Direct and simultaneous identification of Mycobacterium tuberculosis complex (MTBC) and Mycobacterium tuberculosis (MTB) by rapid multiplex nested PCR-ICT assay. J. Microbiol. Methods 66, 440–448.
Soo, P.C., Kung, C.J., Horng, Y.T., Chang, K.C., Lee, J.J., and Peng, W.P. 2012. Detonation nanodiamonds for rapid detection of clinical isolates of Mycobacterium tuberculosis complex in broth culture media. Anal. Chem. 84, 7972–7978.
Stenger, S. 2001. Cytolytic T cells in the immune response to Mycobacterium tuberculosis. Scand. J. Infect. Dis. 33, 483–487.
Stites, S.W., Walters, B., O’Brien-Ladner, A.R., Bailey, K., and Wesselius, L.J. 1998. Increased iron and ferritin content of sputum from patients with cystic fibrosis or chronic bronchitis. Chest 114, 814–819.
Veljkovic Vujaklija, D., Sucic, S., Gulic, T., Dominovic, M., and Rukavina, D. 2012. Cell death mechanisms at the maternal-fetal interface: insights into the role of granulysin. Clin. Dev. Immunol. 2012, 180272.
Vogel, L., Schoonbrood, D., Geluk, F., Hoek, F., Bresser, P., Out, T., Jansen, H., Dankert, J., and van Alphen, L. 1997. Iron-binding proteins in sputum of chronic bronchitis patients with Haemophilus influenzae infections. Eur. Respir. J. 10, 2327–2333.
Wang, H.Y., Kim, H., Kim, S., Kim, D.K., Cho, S.N., and Lee, H. 2015. Performance of a real-time PCR assay for the rapid identification of Mycobacterium species. J. Microbiol. 53, 38–46.
World Health Organization. 2015. Global tuberculosis report 2015. http://www.who.int/tb/publications/global_report/en/.
Yu, F.L., Lee, J.C., Wang, M.S., Hsu, H.L., Chen, T.T., Cheng, C.L., Yang, Y.Y., Wang, G.C., and Yu, M.C. 2016. Evaluation of a modified direct agar proportion method for testing susceptibility of Mycobacterium tuberculosis from MGIT samples. J. Microbiol. Immunol. Infect. 49, 60–65.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplemental material for this article may be found at http://www.springerlink.com/content/120956
Electronic supplementary material
12275_2016_6201_MOESM1_ESM.pdf
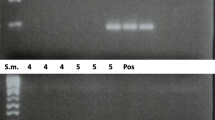
Western blot analysis of IL-10, IFN-γ, IL-25, IL-17, perforin-1, albumin, lactoferrin, ADA, ADA2, granzyme B, granulysin and caspase-1 in sputum samples from five randomly selected patients with TB.
Rights and permissions
About this article
Cite this article
Lai, HC., Horng, YT., Yeh, PF. et al. The assessment of host and bacterial proteins in sputum from active pulmonary tuberculosis. J Microbiol. 54, 761–767 (2016). https://doi.org/10.1007/s12275-016-6201-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-016-6201-x