Abstract
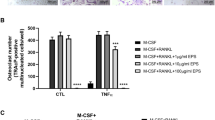
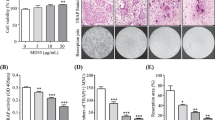
Mammalian γ-glutamyltranspeptidase (GGT) has been identified as a bone-resorbing factor. Since GGT of Bacillus subtilis exhibits similarity in their primary structure and enzymatic characteristics with mammalian GGTs, the bone-resorbing activity of bacterial GGT was examined in this study. Osteoclastogenesis was performed in a co-culture system of mouse calvaria-derived osteoblasts and bone marrow cells. A conditioned medium from GGT-overproducing B. subtilis culture showed significantly higher activity of osteoclast formation than a conditioned medium from wild-type B. subtilis culture. Recombinant GGT (rGGT) of wild-type B. subtilis and an enzymatic activity-defected rGGT of B. subtilis 2288 mutant were expressed in Escherichia coli and purified using His tag. Both purified rGGTs induced similar levels of osteoclastogenesis, suggesting that B. subtilis GGT possesses virulent bone-resorbing activity and its activity is probably independent of its enzymatic activity. Furthermore, a recombinant protein of B. subtilis GGT heavy subunit (Bs rGGT/H) showed strong activity of osteoclastogenesis while the light subunit failed to show strong activity, suggesting that the bone-resorbing activity is mainly located at the heavy subunit. More importantly, the GGT enzymatic activity may not be required for this virulence activity since the light subunit contains the catalytic pocket. In addition, B. subtilis rGGT stimulated mRNA expressions of receptor activator of nuclear factor kappa-B ligand (RANKL) and cyclooxygenase-2 (COX-2), while an osteoprotegerin inhibited the osteoclast formation induced by Bs rGGT/H. This is the first demonstration that bacterial GGT itself is sufficient to act as a bone-resorbing virulence factor via RANKL-dependent pathway. Therefore, it can be hypothesized that GGT of periodontopathic bacteria may play an important role as a virulence factor in bone destruction.
Similar content being viewed by others
References
Brandstrom, H., Bjorkman, T., and Ljunggren, O. 2001. Regulation of osteoprotegerin secretion from primary cultures of human bone marrow stromal cells. Biochem. Biophys. Res. Commun. 280, 831–835.
Choi, H.G., Kim, J.M., Kim, B.J., Yoo, Y.J., and Cha, J.H. 2007. Mouse strain-dependent osteoclastogenesis in response to lipopolysaccharide. J. Microbiol. 45, 566–571.
Hanigan, M.H., Frierson, H.F., Jr., Brown, J.E., Lovell, M.A., and Taylor, P.T. 1994. Human ovarian tumors express gamma-glutamyl transpeptidase. Cancer Res. 54, 286–290.
Hanigan, M.H., Frierson, H.F., Jr., Swanson, P.E., and De Young, B.R. 1999. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum. Pathol. 30, 300–305.
Hofbauer, L.C., Dunstan, C.R., Spelsberg, T.C., Riggs, B.L., and Khosla, S. 1998. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 250, 776–781.
Hofbauer, L.C., Khosla, S., Dunstan, C.R., Lacey, D.L., Boyle, W.J., and Riggs, B.L. 2000. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J. Bone. Miner. Res. 15, 2–12.
Hsu, H., Lacey, D.L., Dunstan, C.R., Solovyev, I., Colombero, A., Timms, E., Tan, H.L., Elliott, G., Kelley, M.J., Sarosi, I., et al. 1999. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc. Natl. Acad. Sci. USA 96, 3540–3545.
Kunst, F., Ogasawara, N., Moszer, I., Albertini, A.M., Alloni, G., Azevedo, V., Bertero, M.G., Bessieres, P., Bolotin, A., Borchert, S., et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390, 249–256.
Lacey, D.L., Timms, E., Tan, H.L., Kelley, M.J., Dunstan, C.R., Burgess, T., Elliott, R., Colombero, A., Elliott, G., Scully, S., et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176.
Lieberman, M.W., Barrios, R., Carter, B.Z., Habib, G.M., Lebovitz, R.M., Rajagopalan, S., Sepulveda, A.R., Shi, Z.Z., and Wan, D.F. 1995. gamma-Glutamyl transpeptidase. What does the organization and expression of a multipromoter gene tell us about its functions? Am. J. Pathol. 147, 1175–1185.
Lin, L.L., Chou, P.R., Hua, Y.W., and Hsu, W.H. 2006. Overexpression, one-step purification, and biochemical characterization of a recombinant gamma-glutamyltranspeptidase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 73, 103–112.
Minami, H., Suzuki, H., and Kumagai, H. 2003a. A mutant Bacillus subtilis gamma-glutamyltranspeptidase specialized in hydrolysis activity. FEMS Microbiol. Lett. 224, 169–173.
Minami, H., Suzuki, H., and Kumagai, H. 2003b. Salt-tolerant γ-glutamyltranspeptidase from Bacillus subtilis 168 with glutaminase activity. Enzyme Microb. Technol. 32, 431–438.
Nagai, M. and Sato, N. 1999. Reciprocal gene expression of osteoclastogenesis inhibitory factor and osteoclast differentiation factor regulates osteoclast formation. Biochem. Biophys. Res. Commun. 257, 719–723.
Niida, S., Kawahara, M., Ishizuka, Y., Ikeda, Y., Kondo, T., Hibi, T., Suzuki, Y., Ikeda, K., and Taniguchi, N. 2004. Gamma-glutamyltranspeptidase stimulates receptor activator of nuclear factor-kappaB ligand expression independent of its enzymatic activity and serves as a pathological bone-resorbing factor. J. Biol. Chem. 279, 5752–5756.
Ogawa, Y., Hosoyama, H., Hamano, M., and Motai, H. 1991. Purification and properties of gamma-glutamyltranspeptidase from Bacillus subtilis (natto). Agric. Biol. Chem. 55, 2971–2977.
Ogawa, Y., Sugiura, D., Motai, H., Yuasa, K., and Tahara, Y. 1997. DNA sequence of Bacillus subtilis (natto) NR-1 gamma-glutamyltranspeptidase gene, ggt. Biosci. Biotechnol. Biochem. 61, 1596–1600.
Simonet, W.S., Lacey, D.L., Dunstan, C.R., Kelley, M., Chang, M.S., Luthy, R., Nguyen, H.Q., Wooden, S., Bennett, L., Boone, T., et al. 1997. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89, 309–319.
Suda, T., Jimi, E., Nakamura, I., and Takahashi, N. 1997. Role of 1 alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 282, 223–235.
Suzuki, H., Kumagai, H., and Tochikura, T. 1986. gamma-Glutamyltranspeptidase from Escherichia coli K-12: formation and localization. J. Bacteriol. 168, 1332–1335.
Takahashi, N., Udagawa, N., and Suda, T. 1999. A new member of tumor necrosis factor ligand family, ODF/OPGL/TRANCE/RANKL, regulates osteoclast differentiation and function. Biochem. Biophys. Res. Commun. 256, 449–455.
Taniguchi, N., House, S., Kuzumaki, N., Yokosawa, N., Yamagiwa, S., Iizuka, S., Makita, A., and Sekiya, C. 1985a. A monoclonal antibody against gamma-glutamyltransferase from human primary hepatoma: its use in enzyme-linked immunosorbent assay of sera of cancer patients. J. Natl. Cancer Inst. 75, 841–847.
Taniguchi, N., Iizuka, S., Zhe, Z.N., House, S., Yokosawa, N., Ono, M., Kinoshita, K., Makita, A., and Sekiya, C. 1985b. Measurement of human serum immunoreactive gamma-glutamyl transpeptidase in patients with malignant tumors using enzyme-linked immunosorbent assay. Cancer Res. 45, 5835–5839.
Tate, S.S. and Meister, A. 1981. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol. Cell. Biochem. 39, 357–368.
Udagawa, N., Takahashi, N., Yasuda, H., Mizuno, A., Itoh, K., Ueno, Y., Shinki, T., Gillespie, M.T., Martin, T.J., Higashio, K., et al. 2000. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141, 3478–3484.
Vidal, O.N., Sjogren, K., Eriksson, B.I., Ljunggren, O., and Ohlsson, C. 1998. Osteoprotegerin mRNA is increased by interleukin-1 alpha in the human osteosarcoma cell line MG-63 and in human osteoblast-like cells. Biochem. Biophys. Res. Commun. 248, 696–700.
Wada, K., Irie, M., Suzuki, H., and Fukuyama, K. 2010. Crystal structure of the halotolerant gamma-glutamyltranspeptidase from Bacillus subtilis in complex with glutamate reveals a unique architecture of the solvent-exposed catalytic pocket. FEBS J. 277, 1000–1009.
Yasuda, H., Shima, N., Nakagawa, N., Mochizuki, S.I., Yano, K., Fujise, N., Sato, Y., Goto, M., Yamaguchi, K., Kuriyama, M., et al. 1998. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139, 1329–1337.
Author information
Authors and Affiliations
Corresponding authors
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, J., Jang, S., Kim, A. et al. Role of bacterial γ-glutamyltranspeptidase as a novel virulence factor in bone-resorbing pathogenesis. J Microbiol. 54, 396–402 (2016). https://doi.org/10.1007/s12275-016-6137-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-016-6137-1