Abstract
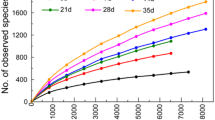
This study examined the fecal bacterial diversity of 15-weekold pigs from three purebred lines: Duroc, Landrace, and Yorkshire. Taxon-dependent and -independent analyses were performed to evaluate differences in the fecal bacterial communities and to identify bacterial genera that can be used to discriminate breeds, following high-throughput pyrosequencing of 16S rRNA genes. Among the breeds evaluated, Landrace had the most diverse bacterial community composition. Prevotella, Blautia, Oscillibacter, and Clostridium were detected in all samples regardless of breed. On the other hand, Catenibacterium, Blautia, Dialister, and Sphaerochaeta were differentially detected among breeds, as demonstrated by the canonical loading plot. The discriminant analysis of principal components plot also showed clear separation of the three purebred pig lines, with a certain degree of similarity between Landrace and Yorkshire pigs and a distinct separation between Duroc pigs and the other two breeds. Other factors not related to breed, such as season or time of sampling and pen effects, may contribute to shaping the gut microbiota of pigs.
Similar content being viewed by others
References
Brodziak, F., Meharg, C., Blaut, M., and Loh, G. 2013. Differences in mucosal gene expression in the colon of two inbred mouse strains after colonization with commensal gut bacteria. PLoS ONE 8, e72317.
Campbell, J.H., Foster, C.M., Vishnivetskaya, T., Campbell, A.G., Yang, Z.K., Wymore, A., Palumbo, V., Chesler, E.J., and Podar, M. 2012. Host genetic and environmental effects on mouse intestinal microbiota. ISME J. 6, 2033–2044.
Chmielowiec-Korzeniowska, A., Tymczyna, L., and Babicz, M. 2012. Assessment of selected parameters of biochemistry, hematology, immunology and production of pigs fattened in different seasons. Archiv. Tierzucht. 5, 469–479.
Chun, J., Lee, J.H., Jung, Y., Kim, M., Kim, S., Kim, B.K., and Lim, Y.W. 2007. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int. J. Syst. Evol. Microbiol. 57, 2259–2261.
de Sevilla, X.F., Fàbrega, E., Tibau, J., and Casellas, J. 2008. Effect of leg conformation on survivability of Duroc, Landrace, and Large White sows. J. Anim. Sci. 86, 2392–2400.
Dowd, S.E., Sun, Y., Wolcott, R.D., Domingo, A., and Carroll, J.A. 2008. Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog. Dis. 5, 459–472.
Gibson, G.R. and Roberfroid, M.B. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125, 1401–1412.
Guixin, Q., Verstegen, M.W.A., and Bosch, M.W. 1995. Variation of digestive capacity between genetically different pig populations: a review. J. Anim. Physiol. Anim. Nutr. 73, 233–242.
Hildebrand, F., Nguyen, T., Brinkman, B., Yunta, R.G., Cauwe, B., Vandenabeele, P., Liston, A., and Raes, J. 2013. Inflammationassociated enterotypes, host genotype, cage and inter-individual effects drive gut microbiota variation in common laboratory mice. Genome Biol. 14, R4.
Ibáñez-Escriche, N., Reixach, J., Lleonart, N., and Noguera, J.L. 2011. Genetic evaluation combining purebred and crossbred data in a pig breeding scheme. J. Anim. Sci. 89, 3881–3889.
Jeon, Y.S., Chun, J., and Kim, B.S. 2013. Identification of household bacterial community and analysis of species shared with human microbiome. Curr. Microbiol. 67, 557–563.
Jeong, J.Y., Park, H.D., Lee, K.H., Weon, H.Y., and Ka, J.O. 2011. Microbial community analysis and identification of alternative host-specific fecal indicators in fecal and river water samples using pyrosequencing. J. Microbiol. 49, 585–594.
Jombart, T. and Ahmed, I. 2011. Adegenet 1.3-1: new tools for the analysis of genome-wide SNP data. Bioinformatics 27, 3070–3071. doi:10.1093/bioinformatics/btr5-1.
Jung, J.Y., Lee, S.H., Kim, J.M., Park, M.S., Bae, J.W., Hahn, Y., Madsen, E.L., and Jeon, C.O. 2011. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl. Environ. Microbiol. 77, 2264–2274.
Kerr, K.R., Forster, G., Dowd, S.E., Ryan, E.P., and Swanson, K.S. 2013. Effects of dietary cooked navy bean on the fecal microbiome of healthy companion dogs. PLoS ONE 8, e74998. doi: 10.1371/journal.pone.0074-98.
Kim, H.B., Borewicz, K., White, B.A., Singer, R.S., Sreevatsan, S., Tu, Z.J., and Isaacson, R.E. 2011. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet. Microbiol. 153, 124–133.
Kim, H.B., Borewicz, K., White, B.A., Singer, R.S., Sreevatsan, S., Tu, Z.J., and Isaacson, R.E. 2012a. Microbial shifts in the swine distal gut in response to the treatment with antimicrobial growth promoter, tylosin. Proc. Natl. Acad. Sci. USA 109, 15485–15490.
Kim, O.S., Cho, Y.J., Lee, K., Yoon, S.H., Kim, M., Na, H., Park, S.C., Jeon, Y.S., Lee, J.H., Yi, H., Won, S., and Chun, J. 2012b. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 62, 716–721.
Kim, O.S., Chae, N., Lim, H.S., Cho, A., and Kim, J.H.. 2012c. Bacterial diversity in ornithogenic soils compared to mineral soils on King George Island, Antarctica. J. Microbiol. 50, 1081–1085.
Kim, T.H., Kim, K.S., Choi, B.H., Yoon, D.H., Jang, G.W., Lee, K.T., Chung, H.Y., Lee, H.Y., Park, H.S., and Lee, J.W. 2005. Genetic structure of pig breeds from Korea and China using microsatellite loci analysis. J. Anim. Sci. 83, 2255–2263.
Lamendella, R., Li, K.C., Oerther, D., and Santo Domingo, J.W. 2013. Molecular diversity of Bacteroidales in fecal and environmental samples and swine-associated subpopulations. Appl. Environ. Microbiol. 79, 816–824.
Ley, R.E., Peterson, D.A., and Gordon, J.I. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124, 837–848.
Loh, G., Brodziak, F., and Blaut, M. 2008. The Toll-like receptors TLR2 and TLR4 do not affect the intestinal microbiota composition in mice. Environ. Microbiol. 10, 709–715.
Lu, X.M., Lu, P.Z., and Zhang, H. 2013. Bacterial communities in manures of piglets and adult pigs bred with different feeds revealed by 16S rDNA 454 pyrosequencing. Appl. Microbiol. Biotechnol. doi: 10.1007/s00253-013-5211-4.
Malmuthuge, N., Griebel, P.J., and Guan, L. 2014. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl. Environ. Microbiol. 80, 2021–2028.
McKnite, A.M., Perez-Munoz, M.E., Lu, L., Williams, E.G., Brewer, S., Andreux, P.A., Bastiaansen, J.W., Wang, X., Kachman, S.D., Auwerx, J., and et al. 2012. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS ONE 7, e39171.
Na, H., Kim, O.K., Yoon, S.H., Kim, Y., and Chun, J. 2011. Comparative approach to capture bacterial diversity of coastal waters. J. Microbiol. 49, 729–740.
O’Hara, A.M. and Shanahan, F. 2006. The gut flora as a forgotten organ. EMBO Rep. 7, 688–693.
Rehman, A., Sina, C., Gavrilova, O., Häsler, R., Ott, S., Baines, J.F., Schreiber, S., and Rosenstiel, P. 2011. Nod2 is essential for temporal development of intestinal microbial communities. Gut 60, 1354–1362.
Shan, T., Reng, Y., Liu, Y., Zhu, L., and Wang, Y. 2010. Breed difference and regulation of the porcine Sirtuin 1 by insulin. J. Anim. Sci. 88, 3909–3917.
Shepherd, M.L., Swecker, W.S., Jensen, R.V., and Ponder, M.A. 2012. Characterization of the fecal bacteria communities of forage- fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol. Lett. 326, 62–68.
Song, Y.G., Shim, S.G., Kim, K.M., Lee, D.H., Kim, D.S., Choi, S.H., Song, J.Y., Kang, H.L., Baik, S.C., Lee, W.K., and et al. 2014. Profiling of the bacteria responsible for pyogenic liver abscess by 16S rRNA gene pyrosequencing. J. Microbiol. 52, 504–509.
Strong, T., Dowd, S., Gutierrez, A.F., and Coffman, J. 2013. Amplicon pyrosequencing of wild duck eubacterial microbiome from a fecal sample reveals numerous species linked to human and animal diseases [v1; ref status: approved with reservations 1, not approved 1, http://f1000r.es/1yy] F1000Research 2013, 2, 224. doi: 10.12688/f1000research.2-224.v1.
Tantasuparuk, W., Lundeheim, N., and Dalin, A.M. 2000. Reproductive performance of purebred Landrace and Yorkshire sows in Thailand with special reference to seasonal influence and parity number. Theriogenology 54, 481–496.
Yan, H., Potu, R., Lu, H., de Almeida, V.V., Stewart, T., Ragland, D., Armstrong, A., Adeola, O., Nakatsu, C.H., and Ajuwon, K.M. 2013. Dietary fat content and fiber type modulate hind gut microbial community and metabolic markers in the pig. PLoS ONE 8, e59581. doi: 10.1371.journal.pone.005913;81.
Yang, L., Bian, G., Su, Y., and Zhu, W. 2014. Comparison of faecal microbial community of lantang, bama, erhualian, meishan, xiaomeishan, duroc, landrace, and yorkshire sows. Asian Australas. J. Anim. Sci. 27, 898–906.
Yu, Z. and Morrison, M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36, 808–812.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Supplemental material for this article may be found at http://www.springerlink.com/content/120956.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pajarillo, E.A.B., Chae, J.P., Balolong, M.P. et al. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J Microbiol. 52, 646–651 (2014). https://doi.org/10.1007/s12275-014-4270-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-014-4270-2