Abstract
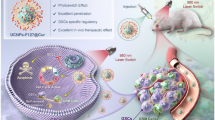
Cell membrane-engineered nano-delivery systems have evolved as a promising strategy to enhance drug bioavailability, offering an alternative for reversing drug resistance in cancer therapy. Herein, a formulated nano-liposome that fabricated by hybridizing cisplatin-resistant A549 cell line (A549/cis) cancer cell membrane and phospholipids for co-delivery of cisplatin and nuclear protein zeste homolog 2 (EZH2)-targeting peptide EIP103, referred to as cLCE, was developed. In vitro results indicated that the formulated nano-liposome can efficiently inhibit A549/cis cancer cell invasion and metastasis through the down-regulation of N-cadherin and vimentin proteins. Mechanistic studies demonstrated that the reduction of nerve growth factor receptor (NGFR) levels and the increase of peroxisome proliferator-activated receptor γ (PPARγ) levels achieved by EIP103 may contribute to the reversal of cisplatin resistance. In vivo results demonstrated that the encapsulation of both cisplatin and EIP103 within cLCE leads to increased intratumoral accumulation and prolonged survival in A549/cis cancer-bearing mice as compared to the individual drugs alone. This can be attributed to the enhanced tumor homing capability of cLCE achieved through the presence of inherited membrane proteins derived from A549/cis cells. Taken together, this study may provide a highly promising therapeutic strategy to improve clinical treatments for cisplatin-resistance non-small-cell lung cancer (NSCLC) as well as other malignant cancers.

Similar content being viewed by others
References
Siegel, R. L.; Miller, K. D.; Wagle, N. S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J Clin. 2023, 73, 17–48
Testa, U.; Castelli, G.; Pelosi, E. Lung cancers: Molecular characterization, clonal heterogeneity and evolution, and cancer stem cells. Cancers 2018, 10, 248.
Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R. L.; Torre, L. A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
Chen, P. X.; Liu, Y. H.; Wen, Y. K.; Zhou, C. C. Non-small cell lung cancer in China. Cancer Commun (Lond). 2022, 42, 937–970
Bethesda, MD: National Cancer Institute. PDQ® Adult Treatment Editorial Board. PDQ Non-small cell lung cancer treatment–Health Professional version Feb. 17, 2023 updated [Online]. Aug. 30, 2023. https://www.cancer.gov/types/lung/hp/non-small-cell-lung-treatment-pdq.
Langer, C. J.; Mok, T.; Postmus, P. E. Targeted agents in the third/fourth-line treatment of patients with advanced (stage III/IV) non-small cell lung cancer (NSCLC). Cancer Treat. Rev. 2013, 39, 252–260.
Rotoli, D.; Santana-Viera, L.; Ibba, M. L.; Esposito, C. L.; Catuogno, S. Advances in oligonucleotide aptamers for NSCLC targeting. Int. J. Mol. Sci. 2020, 21, 6075.
Gridelli, C.; Casaluce, F. Frontline immunotherapy for NSCLC: Alone or not alone. Nat. Rev. Clin. Oncol. 2018, 15, 593–594.
Garon, E. B.; Hellmann, M. D.; Rizvi, N. A.; Carcereny, E.; Leighl, N. B.; Ahn, M. J.; Eder, J. P.; Balmanoukian, A. S.; Aggarwal, C.; Horn, L. et al. Five- year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527.
Stower, H. Effective treatment of NSCLC. Nat. Med. 2020, 26, 1512–1512.
Turner, N. C.; Reis-Filho, J. S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012, 13, e178–e185.
Gettinger, S. N.; Horn, L.; Gandhi, L.; Spigel, D. R.; Antonia, S. J.; Rizvi, N. A.; Powderly, J. D.; Heist, R. S.; Carvajal, R. D.; Jackman, D. M. et al. Overall survival and long-term safety of Nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J. Clin. Oncol. 2015, 33, 2004–2012.
Ardizzoni, A.; Boni, L.; Tiseo, M.; Fossella, F. V.; Schiller, J. H.; Paesmans, M.; Radosavljevic, D.; Paccagnella, A.; Zatloukal, P.; Mazzanti, P. et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: An individual patient data meta-analysis. J. Natl. Cancer Inst. 2007, 99, 847–857.
Vasconcellos, V. F.; Marta, G. N.; Da Silva, E. M.; Gois, A. F.; De Castria, T. B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 1, CD009256.
Chen, S. H.; Chang, J. Y. New insights into mechanisms of cisplatin resistance: From tumor cell to microenvironment. Int. J. Mol. Sci. 2019, 20, 4136.
Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res. 2016, 106, 27–36.
Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014, 5, e1257.
Kim, K. H.; Roberts, C. W. M. Targeting EZH2 in cancer. Nat. Med. 2016, 22, 128–134.
Varambally, S.; Dhanasekaran, S. M.; Zhou, M.; Barrette, T. R.; Kumar-Sinha, C.; Sanda, M. G.; Ghosh, D.; Pienta, K. J.; Sewalt, R. G. A. B.; Otte, A. P. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629.
Huqun; Ishikawa, R.; Zhang, J. L.; Miyazawa, H.; Goto, Y.; Shimizu, Y.; Hagiwara, K.; Koyama, N. Enhancer of zeste homolog 2 is a novel prognostic biomarker in nonsmall cell lung cancer. Cancer 2012, 118, 1599–1606.
Tu, Z. G.; Chen, X. C.; Tian, T.; Chen, G.; Huang, M. J. Prognostic significance of epigenetic regulatory gene expression in patients with non-small-cell lung cancer. Aging 2021, 13, 7397–7415.
Wang, X. H.; Zhao, H. Q.; Lv, L.; Bao, L.; Wang, X.; Han, S. G. Prognostic significance of EZH2 expression in non-small cell lung cancer: A meta-analysis. Sci. Rep. 2016, 6, 19239.
Zang, X. Y.; Gu, J. M.; Zhang, J. Y.; Shi, H.; Hou, S. N.; Xu, X. Y.; Chen, Y. K.; Zhang, Y.; Mao, F.; Qian, H. et al. Exosome-transmitted lncRNA UFC1 promotes non-small-cell lung cancer progression by EZH2-mediated epigenetic silencing of PTEN expression. Cell Death Dis. 2020, 11, 215.
Xu, C. H.; Hao, K. K.; Hu, H. D.; Sheng, Z. H.; Yan, J.; Wang, Q. B.; Yu, L. K. Expression of the enhancer of zeste homolog 2 in biopsy specimen predicts chemoresistance and survival in advanced non-small cell lung cancer receiving first-line platinum-based chemotherapy. Lung Cancer 2014, 86, 268–273.
Liu, X. Y.; Lu, X. Y.; Zhen, F. X.; Jin, S. D.; Yu, T. F.; Zhu, Q.; Wang, W.; Xu, K.; Yao, J. Q.; Guo, R. H. LINC00665 induces acquired resistance to Gefitinib through recruiting EZH2 and activating PI3K/AKT pathway in NSCLC. Mol. Ther. Nucleic Acids 2019, 16, 155–161.
Jiang, M.; Fang, X. C.; Ma, L. L. S.; Liu, M. P.; Chen, M. T.; Liu, J. Y.; Yang, Y. L.; Wang, C. A nucleus-targeting peptide antagonist towards EZH2 displays therapeutic efficacy for lung cancer. Int. J. Pharm. 2022, 622, 121894.
Zhang, M.; Liu, E. G.; Cui, Y. N.; Huang, Y. Z. Nanotechnology-based combination therapy for overcoming multidrug-resistant cancer. Cancer Biol. Med. 2017, 14, 212.
Hu, C. M. J.; Zhang, L. F. Nanoparticle-based combination therapy toward overcoming drug resistance in cancer. Biochem. Pharmacol. 2012, 83, 1104–1111.
Markman, J. L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J. Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879.
Holder, J. E.; Ferguson, C.; Oliveira, E.; Lodeiro, C.; Trim, C. M.; Byrne, L. J.; Bertolo, E.; Wilson, C. M. The use of nanoparticles for targeted drug delivery in non-small cell lung cancer. Front. Oncol. 2023, 13, 1154318.
Chen, J.; Wang, X.; Yuan, Y.; Chen, H. T.; Zhang, L. P.; Xiao, H. H.; Chen, J. Q.; Zhao, Y. X.; Chang, J.; Guo, W. S. et al. Exploiting the acquired vulnerability of cisplatin-resistant tumors with a hypoxia-amplifying DNA repair-inhibiting (HYDRI) nanomedicine. Sci. Adv. 2021, 7, eabc5267.
Chao, M. P.; Weissman, I. L.; Majeti, R. The CD47-SIRPa pathway in cancer immune evasion and potential therapeutic implications. Curr. Opin. Immunol. 2012, 24, 225–232.
Zeng, Y. P.; Li, S. F.; Zhang, S. F.; Wang, L.; Yuan, H.; Hu, F. Q. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B. 2022, 12, 3233–3254.
Long, Y.; Wang, Z.; Fan, J. L.; Yuan, L. Q.; Tong, C. Y.; Zhao, Y. Z.; Liu, B. A hybrid membrane coating nanodrug system against gastric cancer via the VEGFR2/STAT3 signaling pathway. J. Mater. Chem. B 2021, 9, 3838–3855.
Liu, Z. W.; Wang, F. M.; Liu, X. P.; Sang, Y. J.; Zhang, L.; Ren, J. S.; Qu, X. G. Cell membrane-camouflaged liposomes for tumor cell-selective glycans engineering and imaging in vivo. Proc. Natl. Acad. Sci. USA 2021, 118, e2022769118.
Li, X. Y.; Yu, Y. Z.; Chen, Q.; Lin, J. B.; Zhu, X. Q.; Liu, X. T.; He, L. Z.; Chen, T. F.; He, W. L. Engineering cancer cell membrane-camouflaged metal complex for efficient targeting therapy of breast cancer. J. Nanobiotechnol. 2022, 20, 401.
Cao, H. Q.; Dan, Z. L.; He, X. Y.; Zhang, Z. W.; Yu, H. J.; Yin, Q.; Li, Y. P. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 2016, 10, 7738–7748.
Uemura, Y.; Naoi, T.; Kanai, Y.; Kobayashi, K. The efficiency of lipid nanoparticles with an original cationic lipid as a siRNA delivery system for macrophages and dendritic cells. Pharm. Dev. Technol. 2019, 24, 263–268.
Zhou, W.; Wang, J.; Man, W. Y.; Zhang, Q. W.; Xu, W. G. siRNA silencing EZH2 reverses cisplatin-resistance of human non-small cell lung and gastric cancer cells. Asian Pac. J. Cancer Prev. 2015, 16, 2425–2430.
Yang, D. L.; Feng, W. Y.; Zhuang, Y.; Liu, J. X.; Feng, Z. Q.; Xu, T. W.; Wang, W.; Zhu, Y. F.; Wang, Z. X. Long non-coding RNA linc00665 inhibits CDKN1C expression by binding to EZH2 and affects cisplatin sensitivity of NSCLC cells. Mol. Ther. Nucleic Acids 2021, 23, 1053–1065.
Farhood, H.; Serbina, N.; Huang, L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta Biomembr. 1995, 1235, 289–295.
Jääskeläinen, I.; Sternberg, B.; Mönkkönen, J.; Urtti, A. Physicochemical and morphological properties of complexes made of cationic liposomes and oligonucleotides. Int. J. Pharm. 1998, 167, 191–203.
Liskayová, G.; Hubčík, L.; Búcsi, A.; Fazekaš, T.; Martínez, J. C.; Devínsky, F.; Pisárčik, M.; Hanulová, M.; Ritz, S.; Uhríková, D. pH-sensitive N,N-dimethylalkane-1-amine N-oxides in DNA delivery: From structure to transfection efficiency. Langmuir 2019, 35, 13382–13395.
Boulikas, T. Low toxicity and anticancer activity of a novel liposomal cisplatin (Lipoplatin) in mouse xenografts. Oncol. Rep. 2004, 12, 3–12.
Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the epithelial-mesenchymal transition (EMT) with cisplatin resistance. Int. J. Mol. Sci. 2020, 21, 4002.
Cao, Z. Q.; Wang, Z.; Leng, P. Aberrant N-cadherin expression in cancer. Biomed. Pharm. 2019, 118, 109320.
Hui, L. P.; Zhang, S. Y.; Dong, X. J.; Tian, D. L.; Cui, Z. S.; Qiu, X. S. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS One 2013, 8, e62171.
Satelli, A.; Li, S. L. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046.
Kourtidis, A.; Lu, R. F.; Pence, L. J.; Anastasiadis, P. Z. A central role for cadherin signaling in cancer. Exp. Cell Res. 2017, 358, 78–85.
Boldin, M. P.; Goncharov, T. M.; Goltseve, Y. V.; Wallach, D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell 1996, 85, 803–815.
Muzio, M.; Chinnaiyan, A. M.; Kischkel, F. C.; O’Rourke, K.; Shevchenko, A.; Ni, J.; Scaffidi, C.; Bretz, J. D.; Zhang, M.; Gentz, R. et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 1996, 85, 817–827.
Barski, A.; Cuddapah, S.; Cui, K. R.; Roh, T. Y.; Schones, D. E.; Wang, Z. B.; Wei, G.; Chepelev, I.; Zhao, K. J. High-resolution profiling of histone methylations in the human genome. Cell 2007, 129, 823–837.
Dawson, M. A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27.
Zaib, S.; Rana, N.; Khan, I. Histone modifications and their role in epigenetics of cancer. Curr. Med. Chem. 2022, 29, 2399–2411.
Greer, E. L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357.
Margueron, R.; Reinberg, D. The polycomb complex PRC2 and its mark in life. Nature 2012, 469, 343–349.
Moufarrij, S.; Dandapani, M.; Arthofer, E.; Gomez, S.; Srivastava, A.; Lopez-Acevedo, M.; Villagra, A.; Chiappinelli, K. B. Epigenetic therapy for ovarian cancer: Promise and progress. Clin. Epinenet. 2019, 11, 7.
Gao, W.; Zhang, J.; Zhang, M. J.; Hu, L. Y.; Wong, T. S.; Chan, J. Y. W. The functional role of NGFR in regulating chemosensitivity in nasopharyngeal carcinoma. Ann. Oncol. 2018, 29, vii72.
Huang, J.; Yu, Q. H.; Zhou, Y. J.; Chu, Y.; Jiang, F.; Zhu, X. B.; Zhang, J. J.; Wang, Q. A positive feedback loop formed by NGFR and FOXP3 contributes to the resistance of non-small cell lung cancer to icotinib. Transl. Cancer Res. 2020, 9, 1044–1052.
Zhang, J.; Zeng, Y. Y.; Xing, Y. P.; Li, X. R.; Zhou, L. Q.; Hu, L.; Chin, Y. E.; Wu, M. Myristoylation- mediated phase separation of EZH2 compartmentalizes STAT3 to promote lung cancer growth. Cancer Lett. 2021, 516, 84–98.
Lu, Z. H.; Fang, Z.; Guo, Y.; Liu, X. F.; Chen, S. J. Cisplatin resistance of NSCLC cells involves upregulation of visfatin through activation of its transcription and stabilization of mRNA. Chem. Biol. Interact. 2022, 351, 109705.
Mukherjee, T. K.; Paul, K.; Mukhopadhyay, S. Cell signaling molecules as drug targets in lung cancer: An overview. Curr. Opin. Pulm. Med. 2011, 17, 286–291.
Hu, F. F.; Chen, H.; Duan, Y.; Lan, B.; Liu, C. J.; Hu, H.; Dong, X.; Zhang, Q.; Cheng, Y. M.; Liu, M. et al. CBX2 and EZH2 cooperatively promote the growth and metastasis of lung adenocarcinoma. Mol. Ther. Nucleic Acids 2022, 27, 670–684.
Kim, T. W.; Hong, D. W.; Kang, C. M.; Hong, S. H. A novelPPARɣ ligand, PPZ023, overcomes radioresistance via ER stress and cell death in human non-small-cell lung cancer cells. Exp. Mol. Med. 2020, 52, 1730–1743.
Hu, S.; Yu, L. L.; Li, Z. M.; Shen, Y.; Wang, J.; Cai, J.; Xiao, L.; Wang, Z. H. Overexpression of EZH2 contributes to acquired cisplatin resistance in ovarian cancer cells in vitro and in vivo. Cancer Biol. Ther. 2010, 10, 788–795.
Rizzo, S.; Hersey, J. M.; Mellor, P.; Dai, W.; Santos-Silva, A.; Liber, D.; Luk, L.; Titley, I.; Carden, C. P.; Box, G. et al. Ovarian cancer stem cell-like side populations are enriched following chemotherapy and overexpress EZH2. Mol. Cancer Ther. 2011, 10, 325–335.
Dou, D. W.; Ge, X.; Wang, X. X.; Xu, X. D.; Zhang, Z.; Seng, J.; Cao, Z.; Gu, Y. T.; Han, M. L. EZH2 contributes to cisplatin resistance in breast cancer by epigenetically suppressing miR-381 expression. Onco Tarnets Ther. 2019, 12, 9627–9637
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 32101130, 21721002, and 31971295). Financial support from Strategic Priority Research Program of Chinese Academy of Sciences (No. XDB36000000) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, M., Jiang, M., Chen, M. et al. Formulated nano-liposomes for reversal of cisplatin resistance in NSCLC with nucleus-targeting peptide. Nano Res. 16, 12864–12879 (2023). https://doi.org/10.1007/s12274-023-6273-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6273-y