Abstract
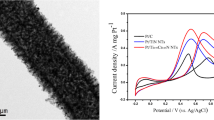
Methanol oxidation reaction (MOR), the key reaction for clean energy generation in fuel cells, is kinetically sluggish and short-lasting because of insufficient catalytic activity and stability of the common Pt-based electrocatalysts. Ordered Pt alloy structures which promise to surmount these issues, are challenging and impractical to fabricate using common high-temperature annealing. To address the urgent need for simple and rapid synthesis methods for such alloys, here we report the versatile plasma-assisted thermal annealing synthesis of a robust electrocatalyst with PtFe alloys supported on N-doped carbon nanotubes (denoted as PtFe@NCNT-P). Benefiting from the reactive plasma-specific effects, the PtFe@NCNT-P electrocatalyst features ultrafine PtFe alloy nanoparticles (mean size ∼ 2.88 nm, ordered degree ∼ 87.07%) and ultrathin N-doped carbon (NC) shells (0.3–0.7 nm), leading to the excellent catalytic activity and stability toward MOR. The catalyst shows the specific and mass activities of 3.99 mA/cm2 and 2,148.5 mA/mg, which are 7.82 and 7.41 times higher than those for commercial Pt/C (0.51 mA/cm2, 290 mA/mg), and 2.18 and 2.59 times higher compared to the plasma-untreated PtFe@NCNT (1.83 mA/cm2, 829.5 mA/mg), respectively. The PtFe@NCNT-P further exhibits extraordinary stability during the long-term chronoamperometry test and 1,000-cycle cyclic voltammetry scanning, much better compared to PtFe@NCNT samples even after the longer thermal annealing. These findings show great potential of the plasma-enabled synthesis of high-performance carbon-supported metallic electrocatalysts for the emerging clean energy technologies.

Similar content being viewed by others
References
Ding, J. J.; Hu, W. L.; Ma, L.; Gan, M. Y.; Xie, F.; Zhan, W.; Lu W. Facile construction of mesoporous carbon enclosed with NiCoPx nanoparticles for desirable Pt-based catalyst support in methanol oxidation. J. Power Sources 2021, 481, 228888.
Li, W.; Wang, D. D.; Zhang, Y. Q.; Tao, L.; Wang, T. H.; Zou, Y. Q.; Wang, Y. Y.; Chen, R.; Wang, S. Y. Defect engineering for fuel-cell electrocatalysts. Adv. Mater. 2020, 32, 1907879.
Neaţu, Ş.; Neaţu, F.; Chirica, I. M.; Borbáth, I.; Tálas, E.; Tompos, A.; Somacescu, S.; Osiceanu, P.; Folgado, M. A.; Chaparro, A. M. et al. Recent progress in electrocatalysts and electrodes for portable fuel cells. J. Mater. Chem. A 2021, 9, 17065–17128.
Tian, H.; Wu, D. X.; Li, J.; Luo, J. M.; Jia, C. M.; Liu, Z. X.; Huang, W.; Chen, Q.; Shim, C. M.; Deng, P. et al. Rational design ternary platinum based electrocatalysts for effective methanol oxidation reaction. J. Energy Chem. 2022, 70, 230–235.
Luo, X. L.; Liu, C.; Wang, X. L.; Shao, Q.; Pi, Y. C.; Zhu, T.; Li, Y. Y.; Huang, X. Q. Spin regulation on 2D Pd−Fe−Pt nanomeshes promotes fuel electrooxidations. Nano Lett. 2020, 20, 1967–1973.
Scofield, M. E.; Koenigsmann, C.; Wang, L.; Liu, H. Q.; Wong, S. S. Tailoring the composition of ultrathin, ternary alloy PtRuFe nanowires for the methanol oxidation reaction and formic acid oxidation reaction. Energy Environ. Sci. 2015, 8, 350–363.
Ren, X. F.; Wang, Y. R.; Liu, A. M.; Zhang, Z. H.; Lv, Q. Y.; Liu, B. H. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306.
Zhu, J. Y.; Chen, S. Q.; Xue, Q.; Li, F. M.; Yao, H. C.; Xu, L.; Chen, Y. Hierarchical porous Rh nanosheets for methanol oxidation reaction. Appl. Catal. B:Environ. 2020, 264, 118520.
Yang, J.; Hübner, R.; Zhang, J. W.; Wan, H.; Zheng, Y. Y.; Wang, H. L.; Qi, H. Y.; He, L. Q.; Li, Y.; Dubale, A. A. et al. A robust PtNi nanoframe/N-doped graphene aerogel electrocatalyst with both high activity and stability. Angew. Chem., Int. Ed. 2021, 60, 9590–9597.
Zou, X.; Chen, S. G.; Wang, Q. M.; Gao, X. Y.; Li, J.; Li, J.; Li, L.; Ding, W.; Wei, Z. D. Leaching- and sintering-resistant hollow or structurally ordered intermetallic PtFe alloy catalysts for oxygen reduction reactions. Nanoscale 2019, 11, 20115–20122.
Xue, S. F.; Deng, W. T.; Yang, F.; Yang, J. L.; Amiinu, I. S.; He, D. P.; Tang, H. L.; Mu, S. C. Hexapod PtRuCu nanocrystalline alloy for highly efficient and stable methanol oxidation. ACS Catal. 2018, 8, 7578–7584.
Lee, D.; Gok, S.; Kim, Y.; Sung, Y. E.; Lee, E.; Jang, J. H.; Hwang, J. Y.; Kwon, O. J.; Lim, T. Methanol tolerant Pt-C core-shell cathode catalyst for direct methanol fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 44588–44596.
Zhao, W.; Ma, L.; Gan, M. Y.; Li, X. D.; Zhang, Y. C.; Hua, X. L.; Wang, L. Engineering intermetallic-metal oxide interface with low platinum loading for efficient methanol electrooxidation. J. Colloid Interface Sci. 2021, 604, 52–60.
Li, X. G.; Bi, W. T.; Zhang, L.; Tao, S.; Chu, W. S.; Zhang, Q.; Luo, Y.; Wu, C. Z.; Xie, Y. Single-atom Pt as co-catalyst for enhanced photocatalytic H2 evolution. Adv. Mater. 2016, 28, 2427–2431.
Zhang, Z. Q.; Liu, J. P.; Wang, J.; Wang, Q.; Wang, Y. H.; Wang, K.; Wang, Z.; Gu, M.; Tang, Z. H.; Lim, J. et al. Single-atom catalyst for high-performance methanol oxidation. Nat. Commun. 2021, 12, 5235.
Zhu, C. Z.; Fu, S. F.; Shi, Q. R.; Du, D.; Lin, Y. H. Single-atom electrocatalysts. Angew. Chem., Int. Ed. 2017, 56, 13944–13960.
Shang, L.; Bian, T.; Zhang, B. H.; Zhang, D. H.; Wu, L. Z.; Tung, C. H.; Yin, Y. D.; Zhang, T. R. Graphene-supported ultrafine metal nanoparticles encapsulated by mesoporous silica: Robust catalysts for oxidation and reduction reactions. Angew. Chem., Int. Ed. 2014, 53, 250–254.
Han, A. L.; Wang, X. J.; Tang, K.; Zhang, Z. D.; Ye, C. L.; Kong, K. J.; Hu, H. B.; Zheng, L. R.; Jiang, P.; Zhao, C. X. et al. An adjacent atomic platinum site enables single-atom iron with high oxygen reduction reaction performance. Angew. Chem., Int. Ed. 2021, 60, 19262–19271.
Zhu, Z. J.; Zhai, Y. L.; Dong, S. J. Facial synthesis of PtM (M = Fe, Co, Cu, Ni) bimetallic alloy nanosponges and their enhanced catalysis for oxygen reduction reaction. ACS Appl. Mater. Interfaces. 2014, 6, 16721–16726.
Deng, J.; Deng, D. H.; Bao, X. H. Robust catalysis on 2D materials encapsulating metals: Concept, application, and perspective. Adv. Mater. 2017, 29, 1606967.
Yoo, T. Y.; Yoo, J. M.; Sinha, A. K.; Bootharaju, M. S.; Jung, E.; Lee, H. S.; Lee, B. H.; Kim, J.; Antink, W. H.; Kim, Y. M. et al. Direct synthesis of intermetallic platinum-alloy nanoparticles highly loaded on carbon supports for efficient electrocatalysis. J. Am. Chem. Soc. 2020, 142, 14190–14200.
Wu, D. F.; Zhang, W.; Lin, A. J.; Cheng, D. J. Low Pt-content ternary PtNiCu nanoparticles with hollow interiors and accessible surfaces as enhanced multifunctional electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 9600–9608.
Xiao, Y. X.; Ying, J.; Tian, G.; Zhang, X. Q.; Janiak, C.; Ozoemena, K. I.; Yang, X. Y. PtPd hollow nanocubes with enhanced alloy effect and active facets for efficient methanol oxidation reaction. Chem. Commun. 2021, 57, 986–989.
Yue, X. Y.; Pu, Y. G.; Zhang, W.; Zhang, T.; Gao, W. Ultrafine Pt nanoparticles supported on double-shelled C/TiO2 hollow spheres material as highly efficient methanol oxidation catalysts. J. Energy Chem. 2020, 49, 275–282.
Ouyang, Y. R.; Cao, H. J.; Wu, H. J.; Wu, D. B.; Wang, F. Q.; Fan, X. J.; Yuan, W. Y.; He, M. X.; Zhang, L. Y.; Li, C. M. Tuning Pt-skinned PtAg nanotubes in nanoscales to efficiently modify electronic structure for boosting performance of methanol electrooxidation. Appl. Catal. B:Environ. 2020, 265, 118606.
Long, X. Y.; Yin, P.; Lei, T.; Wang, K. C.; Zhan, Z. X. Methanol electro-oxidation on Cu@Pt/C core-shell catalyst derived from Cu−MOF. Appl. Catal. B:Environ. 2020, 260, 118187.
Lei, W. J.; Li, M. G.; He, L.; Meng X.; Mu, Z. J.; Yu, Y. S.; Ross, F. M.; Yang, W. W. A general strategy for bimetallic Pt-based nanobranched structures as highly active and stable oxygen reduction and methanol oxidation bifunctional catalysts. Nano Res. 2020, 13, 638–645.
Zhang, Y. P.; Gao, F.; Song, T. X.; Wang, C.; Chen, C. Y.; Du, Y. K. Novel networked wicker-like PtFe nanowires with branch-rich exteriors for efficient electrocatalysis. Nanoscale 2019, 11, 15561–15566.
Li, H. Y.; Wu, X. S.; Tao, X. L.; Lu, Y.; Wang, Y. W. Direct synthesis of ultrathin Pt nanowire arrays as catalysts for methanol oxidation. Small 2020, 16, 2001135.
Li, Z. J.; Jiang, X.; Wang, X. R.; Hu, J. R.; Liu, Y. Y.; Fu, G. T.; Tang, Y. W. Concave PtCo nanocrosses for methanol oxidation reaction. Appl. Catal. B:Environ. 2020, 277, 119135.
Wang, L. J.; Tian, X. L.; Xu, Y. Y.; Zaman, S.; Qi, K.; Liu, H. F.; Xia, B. Y. Engineering one-dimensional and hierarchical PtFe alloy assemblies towards durable methanol electrooxidation. J. Mater. Chem. A 2019, 7, 13090–13095.
Shan, A. X.; Huang, S. Y.; Zhao, H. F.; Jiang, W. G.; Teng, X. A.; Huang, Y. C.; Chen, C.; Wang, R. M.; Lau, W. M. Atomic-scaled surface engineering Ni−Pt nanoalloys towards enhanced catalytic efficiency for methanol oxidation reaction. Nano Res. 2020, 13, 3088–3097.
Yang, C. Z.; Jiang, Q. G.; Liu, H.; Yang, L.; He, H. Y.; Huang, H. J.; Li, W. H. Pt-on-Pd bimetallic nanodendrites stereoassembled on MXene nanosheets for use as high-efficiency electrocatalysts toward the methanol oxidation reaction. J. Mater. Chem. A 2021, 9, 15432–15440.
He, S. Q.; Liu, Y.; Zhan, H. B.; Guan, L. H. Direct thermal annealing synthesis of ordered Pt alloy nanoparticles coated with a thin N-doped carbon shell for the oxygen reduction reaction. ACS Catal. 2021, 11, 9355–9365.
Yang, C. L.; Wang, L. N.; Yin, P.; Liu, J. Y.; Chen, M. X.; Yan, Q. Q.; Wang, Z. S.; Xu, S. L.; Chu, S. Q.; Cui, C. H. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464.
Tu, Y. C.; Ren, P. J.; Deng, D. H.; Bao, X. H. Structural and electronic optimization of graphene encapsulating binary metal for highly efficient water oxidation. Nano Energy 2018, 52, 494–500.
Yu, L.; Deng, D. H.; Bao, X. H. Chain mail for catalysts. Angew. Chem., Int. Ed. 2020, 59, 15294–15297.
Tu, Y. C.; Deng, J.; Ma, C.; Yu, L.; Bao, X. H.; Deng, D. H. Double-layer hybrid chainmail catalyst for high-performance hydrogen evolution. Nano Energy 2020, 72, 104700.
Chen, Y. J.; Ji, S. F.; Wang, Y. G.; Dong, J. C.; Chen, W. X.; Li, Z.; Shen, R. A.; Zheng, L. R.; Zhuang, Z. B.; Wang, D. S. et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2017, 56, 6937–6941.
Deng, J.; Ren, P. J.; Deng, D. H.; Bao, X. H. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2015, 54, 2100–2104.
Cui, X. J.; Ren, P. J.; Deng, D. H.; Deng, J.; Bao, X. H. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 2016, 9, 123–129.
van Deelen, T. W.; Hernández Mejía, C.; de Jong, K. P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300.
Jang, M. H.; Agarwal, R.; Nukala, P.; Choi, D.; Johnson, A. T. C.; Chen, I. W.; Agarwal, R. Observing oxygen vacancy driven electroforming in Pt−TiO2−Pt device via strong metal support interaction. Nano Lett. 2016, 16, 2139–2144.
Lin, G. X.; Ju, Q. J.; Jin, Y.; Qi, X. H.; Liu, W. J.; Huang, F. Q.; Wang, J. C. Suppressing dissolution of Pt-based electrocatalysts through the electronic metal-support interaction. Adv. Energy Mater. 2021, 11, 2101050.
Zhou, Y. W.; Chen, Y. F.; Jiang, K.; Liu, Z.; Mao, Z. J.; Zhang, W. Y.; Lin, W. F.; Cai, W. B. Probing the enhanced methanol electrooxidation mechanism on platinum-metal oxide catalyst. Appl. Catal. B:Environ. 2021, 280, 119393.
Wang, Q.; Wang, X. K.; Chai, Z. F.; Hu, W. P. Low-temperature plasma synthesis of carbon nanotubes and graphene based materials and their fuel cell applications. Chem. Soc. Rev. 2013, 42, 8821–8834.
Dou, S.; Tao, L.; Wang, R. L.; El Hankari, S.; Chen, R.; Wang, S. Y. Plasma-assisted synthesis and surface modification of electrode materials for renewable energy. Adv. Mater. 2018, 30, 1705850.
Ma, Y. L.; Wang, Q.; Miao, Y. L.; Lin, Y.; Li, R. Y. Plasma synthesis of Pt nanoparticles on 3D reduced graphene oxide-carbon nanotubes nanocomposites towards methanol oxidation reaction. Appl. Surf. Sci. 2018, 450, 413–421.
Sun, H. M.; Miao, Y. L.; Wu, T.; Wang, Q. Exfoliation of bimetallic (Ni, Co) carbonate hydroxide nanowires by Ar plasma for enhanced oxygen evolution. Chem. Commun. 2020, 56, 872–875.
Niu, Z. Q.; Becknell, N.; Yu, Y.; Kim, D.; Chen, C.; Kornienko, N.; Somorjai, G. A.; Yang, P. D. Anisotropic phase segregation and migration of Pt in nanocrystals en route to nanoframe catalysts. Nat. Mater. 2016, 15, 1188–1194.
Chung, D. Y.; Jun, S. W.; Yoon, G.; Kwon, S. G.; Shin, D. Y.; Seo, P.; Yoo, J. M.; Shin, H.; Chung, Y. H.; Kim, H. et al. Highly durable and active PtFe nanocatalyst for electrochemical oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 15478–15485.
Wang, Y.; Ren, P. J.; Hu, J. T.; Tu, Y. C.; Gong, Z. M.; Cui, Y.; Zheng, Y. P.; Chen, M. S.; Zhang, W. J.; Ma, C. et al. Electron penetration triggering interface activity of Pt-graphene for CO oxidation at room temperature. Nat. Commun. 2021, 12, 5814.
Luo, X.; Wei, X. Q.; Wang, H. J.; Gu, W. L.; Kaneko, T.; Yoshida, Y.; Zhao, X.; Zhu, C. Z. Secondary-atom-doping enables robust Fe−N−C single-atom catalysts with enhanced oxygen reduction reaction. Nano-Micro Lett. 2020, 12, 163.
Acknowledgements
This research was financially supported by the Anhui Provincial Natural Science Foundation (No. 2208085MA16), the National Natural Science Foundation of China (No. 11575253), the Anhui Provincial key research and development plan (No. 1704a0902017), the Anhui Provincial Natural Science Foundation for Distinguished Young Scholars of China (No. 1608085J03), the Hefei Institutes of Physical Science, Chinese Academy of Sciences Director’s Fund (No. YZJJ201505), and the Key Lab of Photovoltaic and Energy Conservation Materials of Chinese Academy of Sciences (No. PECL2018QN005). K. O. acknowledges partial support from the Australian Research Council (ARC) and QUT Centre for Materials Science.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4890_MOESM1_ESM.pdf
Plasma-enabled synthesis of ordered PtFe alloy nanoparticles encapsulated with ultrathin N-doped carbon shells for efficient methanol electrooxidation
Rights and permissions
About this article
Cite this article
Sun, X., Mao, Z., Wang, R. et al. Plasma-enabled synthesis of ordered PtFe alloy nanoparticles encapsulated with ultrathin N-doped carbon shells for efficient methanol electrooxidation. Nano Res. 16, 2065–2075 (2023). https://doi.org/10.1007/s12274-022-4890-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4890-5