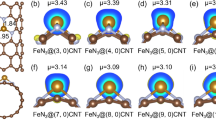
Abstract
Low-coordinated single atom catalysts compared to M-N4 are appealing in optimized electronic structure for CO2 electro-reduction, but the preparation is still very challenging. Herein, a novel single Ni atom catalyst with Ni-N1-C3 configuration is in-situ evolved on curved carbon nanotubes. The obtained Ni-N1-C3 catalyst exhibits a superior CO Faradaic efficiency of 97% and turnover frequency of 2,890 h−1 at −0.9 V versus the reversible hydrogen electrode, as well as long-term stability over 45 h. High current densities exceeding 200 mA·cm−2 and CO Faradaic efficiency of 99% are achieved in flow-cell. Moreover, in-situ potential-and time-dependent Raman spectra identify the key intermediates of *COOH and *CO during CO2-to-CO conversion. Theoretical calculations reveal that the upward-shifted d-band center and charge-rich Ni sites of Ni-N1-C3 facilitate the electron transfer to *COOH and thus reduce the *COOH formation energy barrier. This work demonstrates a strategy for modulating the coordination environment for efficient CO2 reduction.

Similar content being viewed by others
References
Gu, J.; Hsu, C. S.; Bai, L. C.; Chen, H. M.; Hu, X. L. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 2019, 364, 1091–1094.
Dinh, C. T.; Burdyny, T.; Kibria, M. G.; Seifitokaldani, A.; Gabardo, C. M.; De Arquer, F. P. G.; Kiani, A.; Edwards, J. P.; De Luna, P.; Bushuyev, O. S. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 2018, 360, 783–787.
Ren, S. X.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C. P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369.
Nam, D. H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F. W.; Agapie, T.; Peters, J. C.; Shekhah, O.; Eddaoudi, M.; Sargent, E. H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266–276.
Wang, L. M.; Chen, W. L.; Zhang, D. D.; Du, Y. P.; Amal, R.; Qiao, S. Z.; Wu, J. B.; Yin, Z. Y. Surface strategies for catalytic CO2 reduction: From two-dimensional materials to nanoclusters to single atoms. Chem. Soc. Rev. 2019, 48, 5310–5349.
Su, X.; Yang, X. F.; Huang, Y. Q.; Liu, B.; Zhang, T. Single-atom catalysis toward efficient CO2 conversion to CO and formate products. Acc. Chem. Res. 2019, 52, 656–664.
Yang, H. B.; Hung, S. F.; Liu, S.; Yuan, K. D.; Miao, S.; Zhang, L. P.; Huang, X.; Wang, H. Y.; Cai, W. Z.; Chen, R. et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 2018, 3, 140–147.
He, Q.; Lee, J. H.; Liu, D. B.; Liu, Y. M.; Lin, Z. X.; Xie, Z. H.; Hwang, S.; Kattel, S.; Song, L.; Chen, J. G. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst. Adv. Funct. Mater. 2020, 30, 2000407.
Ju, W.; Bagger, A.; Hao, G. P.; Varela, A. S.; Sinev, I.; Bon, V.; Cuenya, B. R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944.
Zhang, Z.; Xiao, J. P.; Chen, X. J.; Yu, S.; Yu, L.; Si, R.; Wang, Y.; Wang, S. H.; Meng, X. G.; Wang, Y. et al. Reaction mechanisms of well-defined metal-N4 sites in electrocatalytic CO2 reduction. Angew. Chem., Int. Ed. 2018, 57, 16339–16342.
Fei, H. L.; Dong, J. C.; Chen, D. L.; Hu, T. D.; Duan, X. D.; Shakir, I.; Huang, Y.; Duan, X. F. Single atom electrocatalysts supported on graphene or graphene-like carbons. Chem. Soc. Rev. 2019, 48, 5207–5241.
Jing, H. Y.; Zhu, P.; Zheng, X. B.; Zhang, Z. D.; Wang, D. S.; Li, Y. D. Theory-oriented screening and discovery of advanced energy transformation materials in electrocatalysis. Adv. Powder Mater. 2022, 1, 100013.
Wang, Y.; Zheng, X. B.; Wang, D. S. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752.
Sun, X. H.; Tuo, Y.; Ye, C. L.; Chen, C.; Lu, Q.; Li, G. N.; Jiang, P.; Chen, S. H.; Zhu, P.; Ma, M. et al. Phosphorus induced electron localization of single iron sites for boosted CO2 electroreduction reaction. Angew. Chem., Int. Ed. 2021, 60, 23614–23618.
Chen, S. H.; Li, W. H.; Jiang, W. J.; Yang, J. R.; Zhu, J. X.; Wang, L. Q.; Ou, H. H.; Zhuang, Z. C.; Chen, M. Z.; Sun, X. H. et al. MOF encapsulating N-heterocyclic carbene-ligated copper single-atom site catalyst towards efficient methane electrosynthesis. Angew. Chem., Int. Ed. 2022, 61, e202114450.
Cheng, Y.; Zhao, S. Y.; Li, H. B.; He, S.; Veder, J. P.; Johannessen, B.; Xiao, J. P.; Lu, S. F.; Pan, J.; Chisholm, M. F. et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2. Appl. Catal. B:Environ. 2019, 243, 294–303.
Jiang, K.; Siahrostami, S.; Zheng, T. T.; Hu, Y. F.; Hwang, S.; Stavitski, E.; Peng, Y. D.; Dynes, J.; Gangisetty, M.; Su, D. et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction. Energy Environ. Sci. 2018, 11, 893–903.
Gong, Y. N.; Jiao, L.; Qian, Y. Y.; Pan, C. Y.; Zheng, L. R.; Cai, X. C.; Liu, B.; Yu, S. H.; Jiang, H. L. Regulating coordination environment of single-atom Ni electrocatalysts templated by MOF for boosting CO2 reduction. Angew. Chem., Int. Ed. 2020, 59, 2705–2709.
Möller, T.; Ju, W.; Bagger, A.; Wang, X. L.; Luo, F.; Thanh, T. N.; Varela, A. S.; Rossmeisl, J.; Strasser, P. Efficient CO2 to CO electrolysis on solid Ni-N-C catalysts at industrial current densities. Energy Environ. Sci. 2019, 12, 640–647.
Zhao, X. H.; Liu, Y. Y. Unveiling the active structure of single nickel atom catalysis: Critical roles of charge capacity and hydrogen bonding. J. Am. Chem. Soc. 2020, 142, 5773–5777.
Hao, Z. J.; Chen, J. X.; Zhang, D. F.; Zheng, L. R.; Li, Y. M.; Yin, Z.; He, G.; Jiao, L.; Wen, Z. H.; Lv, X. J. Coupling effects of Zn single atom and high curvature supports for improved performance of CO2 reduction. Sci. Bull. 2021, 66, 1649–1658.
Liu, D. B.; Li, X. Y.; Chen, S. M.; Yan, H.; Wang, C. D.; Wu, C. Q.; Haleem, Y. A.; Duan, S.; Lu, J. L.; Ge, B. H. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 2019, 4, 512–518.
Zhuang, Z. C.; Li, Y. H.; Yu, R. H.; Xia, L. X.; Yang, J. R.; Lang, Z. Q.; Zhu, J. X.; Huang, J. Z.; Wang, J. O.; Wang, Y. et al. Reversely trapping atoms from a perovskite surface for high-performance and durable fuel cell cathodes. Nat. Catal. 2022, 5, 300–310.
Kwon, K. C.; Suh, J. M.; Varma, R. S.; Shokouhimehr, M.; Jang, H. W. Electrocatalytic water splitting and CO2 reduction: Sustainable solutions via single-atom catalysts supported on 2D materials. Small Methods 2019, 3, 1800492.
Zheng, W. Z.; Yang, J.; Chen, H. Q.; Hou, Y.; Wang, Q.; Gu, M.; He, F.; Xia, Y.; Xia, Z.; Li, Z. J. et al. Atomically defined undercoordinated active sites for highly efficient CO2 electroreduction. Adv. Funct. Mater. 2020, 30, 1907658.
Wu, X. S.; Chen, J. L.; Wang, M.; Li, X. Y.; Yang, L.; Li, G.; Shan, L.; Li, X. Y.; Lin, Y. X.; Jiang, J. High-curvature carbon-supported Ni single atoms with charge polarization for highly efficient CO2 reduction. Chem. Commun. 2022, 58, 2914–2917.
Chen, K. J.; Liu, K.; An, P. D.; Li, H.; Lin, Y. Y.; Hu, J. H.; Jia, C. K.; Fu, J. W.; Li, H. M.; Liu, H. et al. Iron phthalocyanine with coordination induced electronic localization to boost oxygen reduction reaction. Nat. Commun. 2020, 11, 4173.
Ren, W. H.; Tan, X.; Yang, W. F.; Jia, C.; Xu, S. M.; Wang, K. X.; Smith, S. C.; Zhao, C. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2. Angew. Chem., Int. Ed. 2019, 58, 6972–6976.
Yang, F. Q.; Yu, H. M.; Mao, X. Y.; Meng, Q. G.; Chen, S. X.; Deng, Q.; Zeng, Z. L.; Wang, J.; Deng, S. G. Boosting electrochemical CO2 reduction on ternary heteroatoms-doped porous carbon. Chem. Eng. J. 2021, 425, 131661.
Gándara, F.; Uribe-Romo, F. J.; Britt, D. K.; Furukawa, H.; Lei, L.; Cheng, R.; Duan, X. F.; O’Keeffe, M.; Yaghi, O. M. Porous, conductive metal-triazolates and their structural elucidation by the charge-flipping method. Chem. -Eur. J. 2012, 18, 10595–10601.
Zhao, C. M.; Dai, X. Y.; Yao, T.; Chen, W. X.; Wang, X. Q.; Wang, J.; Yang, J.; Wei, S. Q.; Wu, Y. E.; Li, Y. D. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081.
Zhu, Q. C.; Xu, S. M.; Harris, M. M.; Ma, C.; Liu, Y. S.; Wei, X.; Xu, H. S.; Zhou, Y. X.; Cao, Y. C.; Wang, K. X. et al. A composite of carbon-wrapped Mo2C nanoparticle and carbon nanotube formed directly on Ni foam as a high-performance binder-free cathode for Li-O2 batteries. Adv. Funct. Mater. 2016, 26, 8514–8520.
Yin, H. Y.; Zhu, J. J.; Chen, J. L.; Gong, J. Y.; Nie, Q. L. MOF-derived in situ growth of carbon nanotubes entangled Ni/NiO porous polyhedrons for high performance glucose sensor. Mater. Lett. 2018, 221, 267–270.
Hou, Y.; Liang, Y. L.; Shi, P. C.; Huang, Y. B.; Cao, R. Atomically dispersed Ni species on N-doped carbon nanotubes for electroreduction of CO2 with nearly 100% CO selectivity. Appl. Catal. B:Environ. 2020, 271, 118929.
Li, X. G.; Bi, W. T.; Chen, M. L.; Sun, Y. X.; Ju, H. X.; Yan, W. S.; Zhu, J. F.; Wu, X. J.; Chu, W. S.; Wu, C. Z. et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction. J. Am. Chem. Soc. 2017, 139, 14889–14892.
Yang, F. Q.; Jiang, C.; Ma, M. F.; Shu, F. H.; Mao, X. Y.; Yu, W. K.; Wang, J.; Zeng, Z. L.; Deng, S. G. Solid-state synthesis of Cu nanoparticles embedded in carbon substrate for efficient electrochemical reduction of carbon dioxide to formic acid. Chem. Eng. J. 2020, 400, 125879.
Xue, Y. R.; Huang, B. L.; Yi, Y. P.; Guo, Y.; Zuo, Z. C.; Li, Y. J.; Jia, Z. Y.; Liu, H. B.; Li, Y. L. Anchoring zero valence single atoms of nickel and iron on graphdiyne for hydrogen evolution. Nat. Commun. 2011, 9, 1460.
Zou, G. Q.; Hou, H. S.; Foster, C. W.; Banks, C. E.; Guo, T. X.; Jiang, Y. L.; Zhang, Y.; Ji, X. B. Advanced hierarchical vesicular carbon co-doped with S, P, N for high-rate sodium storage. Adv. Sci. 2011, 5, 1800241.
Qiu, H. J.; Ito, Y.; Cong, W. T.; Tan, Y. W.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. W. Nanoporous graphene with single-atom nickel dopants: An efficient and stable catalyst for electrochemical hydrogen production. Angew. Chem., Int. Ed. 2015, 54, 14031–14035.
Fan, Q.; Hou, P. F.; Choi, C.; Wu, T. S.; Hong, S.; Li, F.; Soo, Y. L.; Kang, P.; Jung, Y.; Sun, Z. Y. Activation of Ni particles into single Ni-N atoms for efficient electrochemical reduction of CO2. Adv. Energy Mater. 2020, 10, 1903068.
Wang, X. Q.; Chen, Z.; Zhao, X. Y.; Yao, T.; Chen, W. X.; You, R.; Zhao, C. M.; Wu, G.; Wang, J.; Huang, W. X. et al. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2. Angew. Chem., Int. Ed. 2018, 57, 1944–1948.
Hai, X.; Zhao, X. X.; Guo, N.; Yao, C. H.; Chen, C.; Liu, W.; Du, Y. H.; Yan, H.; Li, J.; Chen, Z. X. et al. Engineering local and global structures of single Co atoms for a superior oxygen reduction reaction. ACS Catal. 2020, 10, 5862–5870.
Yin, P. Q.; Yao, T.; Wu, Y. E.; Zheng, L. R.; Lin, Y.; Liu, W.; Ju, H. X.; Zhu, J. F.; Hong, X.; Deng, Z. X. et al. Single cobalt atoms with precise N-coordination as superior oxygen reduction reaction catalysts. Angew. Chem., Int. Ed. 2016, 55, 10800–10805.
Song, X. K.; Zhang, H.; Yang, Y. Q.; Zhang, B.; Zuo, M.; Cao, X.; Sun, J. H.; Lin, C.; Li, X. P.; Jiang, Z. Bifunctional nitrogen and cobalt codoped hollow carbon for electrochemical syngas production. Adv. Sci. 2018, 5, 1800177.
Koshy, D. M.; Chen, S. C.; Lee, D. U.; Stevens, M. B.; Abdellah, A. M.; Dull, S. M.; Chen, G.; Nordlund, D.; Gallo, A.; Hahn, C. et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni,N-doped carbon catalysts. Angew. Chem., Int. Ed. 2020, 55, 4043–4050.
Zhang, C. H.; Yang, S. Z.; Wu, J. J.; Liu, M. J.; Yazdi, S.; Ren, M. Q.; Sha, J. W.; Zhong, J.; Nie, K. Q.; Jalilov, A. S. et al. Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene. Adv. Energy Mater. 2018, 8, 1703487.
Gao, S.; Jiao, X. C.; Sun, Z. T.; Zhang, W. H.; Sun, Y. F.; Wang, C. M.; Hu, Q. T.; Zu, X. L.; Yang, F.; Yang, S. Y. et al. Ultrathin Co3O4 layers realizing optimized CO2 electroreduction to formate. Angew. Chem., Int. Ed. 2016, 55, 698–702.
Pan, F. P.; Deng, W.; Justiniano, C.; Li, Y. Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction. Appl. Catal. B:Environ. 2018, 226, 463–472.
Lei, F. C.; Liu, W.; Sun, Y. F.; Xu, J. Q.; Liu, K. T.; Liang, L.; Yao, T.; Pan, B. C.; Wei, S. Q.; Xie, Y. Metallic tin quantum sheets confined in graphene toward high-efficiency carbon dioxide electroreduction. Nat. Commun. 2016, 7, 12697.
Zhang, S.; Kang, P.; Meyer, T. J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737.
Shan, W. Y.; Liu, R.; Zhao, H. C.; He, Z. L.; Lai, Y. J.; Li, S. S.; He, G. Z.; Liu, J. F. In situ surface-enhanced Raman spectroscopic evidence on the origin of selectivity in CO2 electrocatalytic reduction. ACS Nano 2020, 14, 11363–11372.
Chernyshova, I. V.; Somasundaran, P.; Ponnurangam, S. On the origin of the elusive first intermediate of CO2 electroreduction. Proc. Natl. Acad. Sci. USA 2018, 115, E9261–E9270.
Pan, Z.; Wang, K.; Ye, K. H.; Wang, Y.; Su, H. Y.; Hu, B. H.; Xiao, J.; Yu, T. W.; Wang, Y.; Song, S. Q. Intermediate adsorption states switch to selectively catalyze electrochemical CO2 reduction. ACS Catal. 2020, 10, 3871–3880.
Yang, Y.; Ohnoutek, L.; Ajmal, S.; Zheng, X. Z.; Feng, Y. Q.; Li, K. J.; Wang, T.; Deng, Y.; Liu, Y. Y.; Xu, D. et al. “Hot edges” in an inverse opal structure enable efficient CO2 electrochemical reduction and sensitive in situ Raman characterization. J. Mater. Chem. A 2019, 7, 11836–11846.
Vasileff, A.; Zhi, X.; Xu, C. C.; Ge, L.; Jiao, Y.; Zheng, Y.; Qiao, S. Z. Selectivity control for electrochemical CO2 reduction by charge redistribution on the surface of copper alloys. ACS Catal. 2019, 9, 9411–9417.
Shang, H. S.; Zhou, X. Y.; Dong, J. C.; Li, A.; Zhao, X.; Liu, Q. H.; Lin, Y.; Pei, J. J.; Li, Z.; Jiang, Z. L. et al. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 2020, 11, 3049.
Acknowledgements
This research work was supported by the National Natural Science Foundation of China (Nos. 21908090, 22008101, 22108243, and 22168023) and the Natural Science Foundation of Jiangxi Province (No. 20212BAB213038). Y. F. acknowledges the 2020 Nanchang University Scholarship for Doctoral Visiting Abroad.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yang, F., Yu, H., Su, Y. et al. Low-coordinated Ni-N1-C3 sites atomically dispersed on hollow carbon nanotubes for efficient CO2 reduction. Nano Res. 16, 146–154 (2023). https://doi.org/10.1007/s12274-022-4623-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4623-9