Abstract
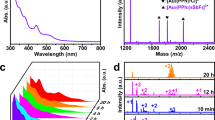
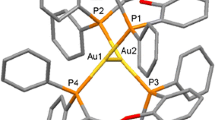
In this study, 1,2-bis(diphenylphosphino)ethane (dppe) ligands are used to synthesize gold nanoclusters with an icosahedral Au13 core. The nanoclusters are characterized and formulated as [Au13(dppe)5Cl2]Cl3 using synchrotron radiation X-ray diffraction, UV/Vis absorption spectroscopy, electrospray ionization mass spectrometry, and density functional theory (DFT) calculations. The bidentate feature of dppe ligands and the positions of coordinating surface gold atoms induce a helical arrangement that forms a propeller-like structure, which reduces the symmetry of the gold nanocluster to C1. Therefore, dppe ligands perform as a directing agent to create chiral an ansa metallamacrocycle [Au13(dppe)5Cl2]3+ nanocluster, as confirmed by simulated electronic circular dichroism spectrum. The highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) gap of the [Au13(dppe)5Cl2]3+ cluster is determined as approx. 1.9 eV, and further confirmed by ultraviolet photoemission spectroscopy analysis and DFT simulation. Furthermore, the photoactivity of [Au13(dppe)5Cl2]3+ is investigated, with the nanocluster shown to possess near-infrared photoluminescence properties, which can be employed for 1O2 photogeneration. The quantum yield of 1O2 photogeneration using the [Au13(dppe)5Cl2]3+ nanocluster is up to 0.71, which is considerably higher than those of anthracene (an organic dye), and Au25 and Au38 nanoclusters.

Similar content being viewed by others
References
Jin, R. C.; Zeng, C. J.; Zhou, M.; Chen, Y. X. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413.
Yamazoe, S.; Koyasu, K.; Tsukuda, T. Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 2014, 47, 816–824.
Fang, J.; Zhang, B.; Yao, Q. F.; Yang, Y.; Xie, J. P.; Yan, N. Recent advances in the synthesis and catalytic applications of ligand-protected, atomically precise metal nanoclusters. Coord. Chem. Rev. 2016, 322, 1–29.
Li, G.; Jin, R. C. Atomically precise gold nanoclusters as new model catalysts. Acc. Chem. Res. 2013, 46, 1749–1758.
Pensa, E.; Cortés, E.; Corthey, G.; Carro, P.; Vericat, C.; Fonticelli, M. H.; Benitez, G.; Rubert, A. A.; Salvarezza, R. C. The chemistry of the sulfur–gold interface: In search of a unified model. Acc. Chem. Res. 2012, 45, 1183–1192.
Yu, Y.; Luo, Z. T.; Chevrier, D. M.; Leong, D. T.; Zhang, P.; Jiang, D. E.; Xie, J. P. Identification of a highly luminescent Au22(SG)18 nanocluster. J. Am. Chem. Soc. 2014, 136, 1246–1249.
Liu, P. X.; Qin, R. X.; Fu, G.; Zheng, N. F. Surface coordination chemistry of metal nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131.
Li, G.; Abroshan, H.; Liu, C.; Zhuo, S.; Li, Z. M.; Xie, Y.; Kim, H. J.; Rosi, N. L.; Jin, R. C. Tailoring the electronic and catalytic properties of Au25 nanoclusters via ligand engineering. ACS Nano 2016, 10, 7998–8005.
Mingos, D. M. P. Gold Clusters, Colloids and Nanoparticles I; Springer: Switzerland, 2014.
Zeng, C. J.; Chen, Y. X.; Kirschbaum, K.; Lambright, K. J.; Jin, R. C. Emergence of hierarchical structural complexities in nanoparticles and their assembly. Science 2016, 354, 1580–1584.
Qian, H. F.; Eckenhoff, W. T.; Zhu, Y.; Pintauer, T.; Jin, R. C. Total structure determination of thiolate-protected Au38 nanoparticles. J. Am. Chem. Soc. 2010, 132, 8280–8281.
Crasto, D.; Malola, S.; Brosofsky, G.; Dass, A.; Häkkinen, H. Single crystal XRD structure and theoretical analysis of the chiral Au30S(S-t-Bu)18 cluster. J. Am. Chem. Soc. 2014, 136, 5000–5005.
Chen, J.; Zhang, Q. F.; Bonaccorso, T. A.; Williard, P. G.; Wang, L. S. Controlling gold nanoclusters by diphospine ligands. J. Am. Chem. Soc. 2014, 136, 92–95.
Lopez-Acevedo, O.; Tsunoyama, H.; Tsukuda, T.; Häkkinen, H.; Aikens, C. M. Chirality and electronic structure of the thiolate-protected Au38 nanocluster. J. Am. Chem. Soc. 2010, 132, 8210–8218.
McKenzie, L. C.; Zaikova, T. O.; Hutchison, J. E. Structurally similar triphenylphosphine-stabilized undecagolds, Au11(PPh3)7Cl3 and [Au11(PPh3)8Cl2]Cl, exhibit distinct ligand exchange pathways with glutathione. J. Am. Chem. Soc. 2014, 136, 13426–13435.
Gutrath, B. S.; Englert, U.; Wang, Y. T.; Simon, U. A missing link in undecagold cluster chemistry: Single-crystal X-ray analysis of [Au11(PPh3)7Cl3]. Eur. J. Inorg. Chem. 2013, 2013, 2002–2006.
Yan, J. Z.; Su, H. F.; Yang, H. Y.; Hu, C. Y.; Malola, S.; Lin, S. C.; Teo, B. K.; Häkkinen, H.; Zheng, N. F. Asymmetric synthesis of chiral bimetallic [Ag28Cu12(SR)24]4–nanoclusters via ion pairing. J. Am. Chem. Soc. 2016, 138, 12751–12754.
Wan, X. K.; Yuan, S. F.; Lin, Z. W.; Wang, Q. M. A chiral gold nanocluster Au20 protected by tetradentate phosphine ligands. Angew. Chem., Int. Ed. 2014, 53, 2923–2926.
Qian, H. F.; Zhu, M. Z.; Gayathri, C.; Gil, R. R.; Jin, R. C. Chirality in gold nanoclusters probed by NMR spectroscopy. ACS Nano 2011, 5, 8935–8942.
Zhu, M. Z.; Qian, H. F.; Meng, X. M.; Jin, S. S.; Wu, Z. K.; Jin, R. C. Chiral Au25 nanospheres and nanorods: Synthesis and insight into the origin of chirality. Nano Lett. 2011, 11, 3963–3969.
Xu, Q.; Kumar, S.; Jin, S. S.; Qian, H. F.; Zhu, M. Z.; Jin, R. C. Chiral 38-gold-atom nanoclusters: Synthesis and chiroptical properties. Small 2014, 10, 1008–1014.
Sheldrick, G. M. SHELXT-integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8.
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341.
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic-behavior. Phys. Rev. A 1988, 38, 3098–3100.
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652.
Burant, J. C.; Scuseria, G. E.; Frisch, M. J. A linear scaling method for hartree-fock exchange calculations of large molecules. J. Chem. Phys. 1996, 105, 8969–8972.
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A. et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009.
C650H600P50Cl10Au65, M r = 23,117.76, monoclinic, space group C/c, a = 29.113(6) Å, b = 29.569(6) Å, c = 70.683(14) Å, β = 101.61(3)°, V = 59602(21) Å3, Z = 4, T = 293(2) K, 81,277 reflections measured, R 1(final) = 0.0830, wR 2 = 0.1931. CCDC-1548942, contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Briant, C. E.; Theobald, B. R. C.; White, J. W.; Bell, L. K.; Mingos, D. M. P.; Welch, A. J. Synthesis and X-ray structural characterization of the centred icosahedral gold cluster compound [Au13(PMe2Ph)10Cl2](PF6)3: The realization of a theoretical prediction. J. Chem. Soc., Chem. Commun. 1981, 201–202.
Shichibu, Y.; Konishi, K. HCl-induced nuclearity convergence in diphosphine-protected ultrasmall gold clusters: A novel synthetic route to “magic-number” Au13 clusters. Small 2010, 6, 1216–1220.
Shichibu, Y.; Suzuki, K.; Konishi, K. Facile synthesis and optical properties of magic-number Au13 clusters. Nanoscale 2012, 4, 4125–4129.
Sugiuchi, M.; Shichibu, Y.; Nakanishi, T.; Hasegawa, Y.; Konishi, K. Cluster–π electronic interaction in a superatomic Au13 cluster bearing σ-bonded acetylide ligands. Chem. Commun. 2015, 51, 13519–13522.
Flack, H. D.; Bernardinelli, G. Absolute structure and absolute configuration. Acta Cryst. 1999, A55, 908–915.
Yamamoto, Y.; Konno, H. Ylide-metal complexes. X. An X-ray photoelectron spectroscopic study of triphenylmethylenephosphorane and gold- and copper-phosphorane complexes. Bull. Chem. Soc. Jpn. 1986, 59, 1327–1330.
Dolamic, I.; Knoppe, S.; Dass, A.; Bürgi, T. First enantioseparation and circular dichroism spectra of Au38 clusters protected by achiral ligands. Nat. Commun. 2012, 3, 798..
Dolamic, I.; Varnholt, B.; Bürgi, T. Chirality transfer from gold nanocluster to adsorbate evidenced by vibrational circular dichroism. Nat. Commun. 2015, 6, 7117..
Zhang, J. W.; Luo, J. H.; Wang, P. M.; Ding, B.; Huang, Y. C.; Zhao, Z. L.; Zhang, J.; Wei, Y. G. Step-by-step strategy from achiral precursors to polyoxometalates-based chiral organic-inorganic hybrids. Inorg. Chem. 2015, 54, 2551–2559.
Zhang, J. W.; Huang, Y. C.; Zhang, J.; She, S.; Hao, J.; Wei, Y. G. A direct anchoring of Anderson-type polyoxometalates in aqueous media with tripodal ligands especially containing the carboxyl group. Dalton Trans. 2014, 43, 2722–2725.
He, X.; Wang, Y. C.; Jiang, H.; Zhao, L. Structurally well-defined sigmoidal gold clusters: Probing the correlation between metal atom arrangement and chiroptical response. J. Am. Chem. Soc. 2016, 138, 5634–5643.
Takano, S.; Tsukuda, T. Amplification of the optical activity of gold clusters by the proximity of BINAP. J. Phys. Chem. Lett. 2016, 7, 4509–4513.
Ji, M.; Gu, X.; Li, X.; Gong, X. G.; Li, J.; Wang, L. S. Experimental and theoretical investigation of the electronic and geometrical structures of the Au32 cluster. Angew. Chem., Int. Ed. 2005, 44, 7119–7123.
Liu, J.; Krishna, K. S.; Losovyj, Y. B.; Chattopadhyay, S.; Lozova, N.; Miller, J. T.; Spivey, J. J.; Kumar, C. S. S. R. Ligand-stabilized and atomically precise gold nanocluster catalysis: A case study for correlating fundamental electronic properties with catalysis. Chem.—Eur. J. 2013, 19, 10201–10208.
Losovyj, Y. B.; Li, S.-C.; Lozova, N.; Katsiev, K.; Stellwagen, D.; Diebold, U.; Kong, L.; Kumar, C. S. S. R. Evidence for s–d hybridization in Au38 clusters. J. Phys. Chem. C 2012, 116, 5857–5861.
Kawasaki, H.; Kumar, S.; Li, G.; Zeng, C. J.; Kauffman, D. R.; Yoshimoto, J.; Iwasaki, Y.; Jin, R. C. Generation of singlet oxygen by photoexcited Au25(SR)18 clusters. Chem. Mater. 2014, 26, 2777–2788.
Li, Z. M.; Liu, C.; Abroshan, H.; Kauffman, D. R.; Li, G. Au38S2(SAdm)20 photocatalyst for one-step selective aerobic oxidations. ACS Catal. 2017, 7, 3368–3374
Zhou, Y.; Li, G. A critical review on carbon-carbon coupling over ultra-small gold nanoclusters. Acta Phys.-Chim. Sin. 2017, 33, 1297–1309.
Chen, H. J.; Liu, C.; Wang, M.; Zhang, C. F.; Luo, N. C.; Wang, Y. H.; Abroshan, H.; Li, G.; Wang, F. Visible light gold nanocluster photocatalyst: Selective aerobic oxidation of amines to imines. ACS Catal. 2017, 7, 3632–3638.
Zhang, C. L.; Chen, Y. D.; Wang, H.; Li, Z.; Zheng, K.; Li, S.; Li, G. Transition-metal-mediated catalytic properties of CeO2-supported gold clusters in aerobic alcohol oxidation. Nano Res., in press, https://doi.org/10.1007/s12274-017-1831-9.
Liu, C.; Zhang, J.; Huang, J.; Zhang, C.; Hong, F.; Zhou, Y.; Li, G.; Haruta, M. Efficient aerobic oxidation of glucose to gluconic acid over activated carbon-supported gold clusters. ChemSuSChem 2017, 10, 1976–1980.
Hayyan, M.; Hashim, M. A.; Al Nashef, I. M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085.
Li, Y. Y.; Cheng, H.; Yao, T.; Sun, Z. H.; Yan, W. S.; Jiang, Y.; Xie, Y.; Sun, Y. F.; Huang, Y. Y.; Liu, S. J. et al. Hexane-driven icosahedral to cuboctahedral structure transformation of gold nanoclusters. J. Am. Chem. Soc. 2012, 134, 17997–18003.
Chen, Y. D.; Zhang, C. L.; Yang, C. P.; Zhang, J. W.; Zheng, K.; Fang, Q. H.; Li, G. A Waugh type [CoMo9O32]6–cluster with atomically dispersed CoIV originates from anderson type [CoMo6O24]3– for photocatalytic oxygen molecule activation. Nanoscale 2017, 9, 15332–15339.
Kurashige, W.; Negishi, Y. Synthesis, stability, and photoluminescence properties of PdAu10(PPh3)8Cl2 clusters. J. Clust. Sci. 2012, 23, 365–374.
Acknowledgements
G. L. acknowledges financial supports by the fund of the National Natural Science Foundation of China (No. 21701168) and the Liaoning Natural Science Foundation (No. 20170540897), and beamline BL14B (Shanghai Synchrotron Radiation Facility) for providing the beam time. A portion of this report was prepared as an account of work sponsored by an agency of the United States Government (D. R. K.). Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Zhou, Y., Zheng, K. et al. Diphosphine-induced chiral propeller arrangement of gold nanoclusters for singlet oxygen photogeneration. Nano Res. 11, 5787–5798 (2018). https://doi.org/10.1007/s12274-017-1935-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1935-2