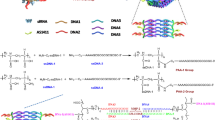
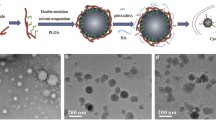
Abstract
Ribonucleic acid (RNA) interference (RNAi) therapies are promising cancer treatment modalities that can specifically target abnormal proto-oncogenes, thus improving the therapeutic effect. For the treatment of pancreatic cancer, targeting one mutant proto-oncogene by RNAi usually does not yield the desired therapeutic efficiency. Both K-ras gene mutations and Notch1 overexpression are common symptoms in pancreatic cancer patients, and play a crucial role in pancreatic cancer cell drug resistance. In this study, biodegradable charged polyester-based vectors (BCPVs) were synthesized for the co-delivery of K-ras and Notch1 small interfering ribonucleic acid (siRNA) into MiaPaCa-2 cells (pancreatic cancer cell line) to overcome drug resistance to gemcitabine (GEM), a first-line chemotherapeutic drug used in the clinic. BCPVs could effectively absorb negative siRNA to form a capsule-like structure, prevent siRNA from nuclease digestion in the serum, and promote effective siRNA cell internalization and endosomal escape. Through K-ras and Notch1 gene silencing in MiaPaCa-2 cells, BCPV-siRNAK-ras-siRNANotch1 nanocomplexes effectively reversed the epithelia-mesenchymal transition (EMT) in MiaPaCa-2 cells, thereby greatly enhancing the sensitivity of MiaPaCa-2 cells to GEM. MiaPaCa-2 cell proliferation, migration, and invasion were effectively inhibited, and cell apoptosis was also significantly enhanced by the synergistic antitumor effect of BCPV-siRNAK-ras-siRNANotch1 nanocomplexes and GEM. These results suggest that this combination RNAi therapy can be used to improve cancer cell sensitivity to chemotherapeutic drugs. Specifically, this newly developed strategy has a great potential for treating pancreatic cancer.

Similar content being viewed by others
References
Siegel, R.; Ma, J. M.; Zou, Z. H.; Jemal, A. Cancer statistics, 2014. CACancer J.Clin. 2014, 64, 9–29.
Siegel, R. L.; Miller, K. D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30.
Waddell, N.; Pajic, M.; Patch, A. M.; Chang, D. K.; Kassahn, K. S.; Bailey, P.; Johns, A. L.; Miller, D.; Nones, K.; Quek, K. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501.
Fleming, J. B.; Shen, G. L.; Holloway, S. E.; Davis, M.; Brekken, R. A. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: Justification for K-rasdirected therapy. Mol. Cancer Res. 2005, 3, 413–423.
Yang, C. B.; Hu, R.; Anderson, T.; Wang, Y. C.; Lin, G. M.; Law, W. C.; Lin, W. J.; Nguyen, Q. T.; Toh, H. T.; Yoon, H. S. et al. Biodegradable nanoparticle-mediated K-ras down regulation for pancreatic cancer gene therapy. J. Mater. Chem. B 2015, 3, 2163–2172.
Vakiani, E.; Solit, D. B. KRAS and BRAF: Drug targets and predictive biomarkers. J. Pathol. 2011, 223, 220–230.
Weijzen, S.; Rizzo, P.; Braid, M.; Vaishnav, R.; Jonkheer, S. M.; Zlobin, A.; Osborne, B. A.; Gottipati, S.; Aster, J. C.; Hahn, W. C. et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human ras-transformed cells. Nat. Med. 2002, 8, 979–986.
Miyamoto, Y.; Maitra, A.; Ghosh, B.; Zechner, U.; Argani, P.; Iacobuzio-Donahue, C. A.; Sriuranpong, V.; Iso, T.; Meszoely, I. M.; Wolfe, M. S. et al. Notch mediates TGFa-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 2003, 3, 565–576.
Bao, B.; Wang, Z. W.; Ali, S.; Kong, D. J.; Li, Y. W.; Ahmad, A.; Banerjee, S.; Azmi, A. S.; Miele, L.; Sarkar, F. H. Notch-1 induces epithelial–mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011, 307, 26–36.
Avila, J. L.; Kissil, J. L. Notch signaling in pancreatic cancer: Oncogene or tumor suppressor? Trends Mol. Med. 2013, 19, 320–327.
De La J.P.; Emerson, L. L.; Goodman, J. L.; Froebe, S. C.; Illum, B. E.; Curtis, A. B.; Murtaugh, L. C. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl. Acad. Sci. USA 2008, 105, 18907–18912.
Yabuuchi, S.; Pai, S. G.; Campbell, N. R.; de Wilde, R. F.; De Oliveira, E.; Korangath, P.; Streppel, M. M.; Rasheed, Z. A.; Hidalgo, M.; Maitra, A. et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013, 335, 41–51.
Wang, Z. W.; Banerjee, S.; Li, Y. W.; Rahman, K. M. W.; Zhang, Y. X.; Sarkar, F. H. Down-regulation of Notch-1 inhibits invasion by inactivation of nuclear factor-κB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006, 66, 2778–2784.
Ji, Z. Y.; Mei, F. C.; Xie, J. W.; Cheng, X. D. Oncogenic KRAS activates hedgehog signaling pathway in pancreatic cancer cells. J. Biol. Chem. 2007, 282, 14048–14055.
Collins, M. A.; Brisset, J. C.; Zhang, Y. Q.; Bednar, F.; Pierre, J.; Heist, K. A.; Galbán, C. J.; Galbán, S.; di Magliano, M. P. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLoS One 2012, 7, e49707.
Burris, H. A.; Moore, M. J.; Andersen, J.; Green, M. R.; Rothenberg, M. L.; Modiano, M. R.; Cripps, M. C.; Portenoy, R. K.; Storniolo, A. M.; Tarassoff, P. et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: Arandomized trial. J. Clin. Oncol. 1997, 15, 2403–2413.
Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J. L.; Gourgou-Bourgade, S.; de la Fouchardière, C. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825.
Mittal, A.; Chitkara, D.; Behrman, S. W.; Mahato, R. I. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials 2014, 35, 7077–7087.
Lima, C. M. S. R.; Savarese, D.; Bruckner, H.; Dudek, A.; Eckardt, J.; Hainsworth, J.; Yunus, F.; Lester, E.; Miller, W.; Saville, W. et al. Irinotecan plus gemcitabine induces both radiographic and CA19–9 tumor marker responses in patients with previously untreated advanced pancreatic cancer. J. Clin. Oncol. 2002, 20, 1182–1191.
Berlin, J. D.; Adak, S.; Vaughn, D. J.; Flinker, D.; Blaszkowsky, L.; Harris, J. E.; Al Benson, B. III. A phase II study of gemcitabine and 5-fluorouracil in metastatic pancreatic cancer: An eastern cooperative oncology group study (E3296). Oncology 2000, 58, 215–218.
Lin, G. M.; Hu, R.; Law, W. C.; Chen, C. K.; Wang, Y. C.; Li, C. H.; Nguyen, Q. T.; Lai, C. K.; Yoon, H. S.; Wang, X. M. et al. Biodegradable nanocapsules as siRNA carriers for mutant K-ras gene silencing of human pancreatic carcinoma cells. Small 2013, 9, 2757–2763.
Lin, G. M.; Yang, C. B.; Hu, R.; Chen, C. K.; Law, W. C.; Anderson, T.; Zhang, B. T.; Nguyen, Q. T.; Toh, H. T.; Yoon, H. S. et al. Interleukin-8 gene silencing on pancreatic cancer cells using biodegradable polymer nanoplexes. Biomater. Sci. 2014, 2, 1007–1015.
Zheng, N.; Song, Z. Y.; Liu, Y.; Zhang, R. J.; Zhang, R. Y.; Yao, C.; Uckun, F. M.; Yin, L. C.; Cheng, J. J. Redoxresponsive, reversibly-crosslinked thiolated cationic helical polypeptides for efficient siRNA encapsulation and delivery. J. Control. Release 2015, 205, 231–239.
Wang, Z. W.; Li, Y. W.; Kong, D. J.; Banerjee, S.; Ahmad, A.; Azmi, A. S.; Ali, S.; Abbruzzese, J. L.; Gallick, G. E.; Sarkar, F. H. Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009, 69, 2400–2407.
Liu, C. X.; Zhao, G.; Liu, J.; Ma, N. C.; Chivukula, P.; Perelman, L.; Okada, K.; Chen, Z. Y.; Gough, D.; Yu, L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J.Control. Release 2009, 140, 277–283.
Birmingham, A.; Anderson, E.; Sullivan, K.; Reynolds, A.; Boese, Q.; Leake, D.; Karpilow, J.; Khvorova, A. A protocol for designing siRNAs with high functionality and specificity. Nat. Protoc. 2007, 2, 2068–2078.
Conde, J.; Ambrosone, A.; Hernandez, Y.; Tian, F. R.; McCully, M.; Berry, C. C.; Baptista, P. V.; Tortiglione, C.; de la Fuente, J. M. 15 years on siRNA delivery: Beyond the state-of-the-art on inorganic nanoparticles for RNAi therapeutics. Nano Today 2015, 10, 421–450.
Wang, J.; Lu, Z.; Wientjes, M. G.; Au, J. L. S. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010, 12, 492–503.
Kanasty, R.; Dorkin, J. R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977.
Zhao, X.; Li, F.; Li, Y. Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G. J.; Hao, J. H. Co-delivery of HIF1a siRNA and gemcitabine via biocompatible lipid-polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25.
Ozcan, G.; Ozpolat, B.; Coleman, R. L.; Sood, A. K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119.
Davidson, B. L.; McCray, P. B. Current prospects for RNA interference-based therapies. Nat. Rev. Gen. 2011, 12, 329–340.
Petrocca, F.; Lieberman, J. Promise and challenge of RNA interference-based therapy for cancer. J. Clin. Oncol. 2011, 29, 747–754.
Yang, C. B.; Panwar, N.; Wang, Y. C.; Zhang, B. T.; Liu, M. X.; Toh, H.; Yoon, H. S.; Tjin, S. C.; Chong, P. H. J.; Law, W. C. et al. Biodegradable charged polyester-based vectors (BCPVs) as an efficient non-viral transfection nanoagent for gene knockdown of the BCR-ABL hybrid oncogene in a human chronic myeloid leukemia cell line. Nanoscale 2016, 8, 9405–9416.
Jones, C. H.; Chen, C. K.; Jiang, M.; Fang, L.; Cheng, C.; Pfeifer, B. A. Synthesis of cationic polylactides with tunable charge densities as nanocarriers for effective gene delivery. Mol. Pharmaceutics 2013, 10, 1138–1145.
Wang, Y. C.; Wu, B.; Yang, C. B.; Liu, M. X.; Sum, T. C.; Yong, K. T. Synthesis and characterization of Mn: ZnSe/ZnS/ZnMnS sandwiched QDs for multimodal imaging and theranostic applications. Small 2016, 12, 534–546.
Ngamcherdtrakul, W.; Morry, J.; Gu, S. D.; Castro, D. J.; Goodyear, S. M.; Sangvanich, T.; Reda, M. M.; Lee, R.; Mihelic, S. A.; Beckman, B. L. et al. Cationic polymer modified mesoporous silica nanoparticles for targeted siRNA delivery to HER2+ breast cancer. Adv. Funct. Mater. 2015, 25, 2646–2659.
Song, L. L.; Peng, Y.; Yun, J.; Rizzo, P.; Chaturvedi, V.; Weijzen, S.; Kast, W. M.; Stone, P. J. B.; Santos, L.; Loredo, A. et al. Notch-1 associates with IKKa and regulates IKK activity in cervical cancer cells. Oncogene 2008, 27, 5833–5844.
Yang, C.; Mo, X.; Lv, J.; Liu, X.; Yuan, M.; Dong, M.; Li, L.; Luo, X.; Fan, X.; Jin, Z. Lipopolysaccharide enhances FcεRI-mediated mast cell degranulation by increasing Ca2+ entry through store-operated Ca2+ channels: Implications for lipopolysaccharide exacerbating allergic asthma. Exp. Physiol. 2012, 97, 1315–1327.
Panwar, N.; Yang, C. B.; Yin, F.; Yoon, H. S.; Chuan, T. S.; Yong, K. T. RNAi-based therapeutic nanostrategy: IL-8 gene silencing in pancreatic cancer cells using gold nanorods delivery vehicles. Nanotechnology 2015, 26, 365101.
Yin, F.; Yang, C. B.; Wang, Q. Q.; Zeng, S. W.; Hu, R.; Lin, G. M.; Tian, J. L.; Hu, S. Y.; Lan, R. F.; Yoon, H. S. et al. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833.
Lennon, A. M.; Wolfgang, C. L.; Canto, M. I.; Klein, A. P.; Herman, J. M.; Goggins, M.; Fishman, E. K.; Kamel, I.; Weiss, M. J.; Diaz, L. A. et al. The early detection of pancreatic cancer: What will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014, 74, 3381–3389.
Anderson, T.; Hu, R.; Yang, C. B.; Yoon, H. S.; Yong, K. T. Pancreatic cancer gene therapy using an siRNA-functionalized single walled carbon nanotubes (SWNTs) nanoplex. Biomater. Sci. 2014, 2, 1244–1253.
Hu, R.; Yang, C. B.; Wang, Y. C.; Lin, G. M.; Qin, W.; Ouyan, Q. L.; Law, W. C.; Nguyen, Q. T.; Yoon, H. S.; Wang, X. M. et al. Aggregation-induced emission (AIE) dye loaded polymer nanoparticles for gene silencing in pancreatic cancer and their in vitro and in vivo biocompatibility evaluation. Nano Res. 2015, 8, 1563–1576.
Li, L.; Gu, W. Y.; Liu, J.; Yan, S. Y.; Xu, Z. P. Aminefunctionalized SiO2 nanodot-coated layered double hydroxide nanocomposites for enhanced gene delivery. Nano Res. 2015, 8, 682–694.
McCully, M.; Hernandez, Y.; Conde, J.; Baptista, P. V.; de la Fuente, J. M.; Hursthouse, A.; Stirling, D.; Berry, C. C. Significance of the balance between intracellular glutathione and polyethylene glycol for successful release of small interfering RNA from gold nanoparticles. Nano Res. 2015, 8, 3281–3292.
Aied, A.; Greiser, U.; Pandit, A.; Wang, W. X. Polymer gene delivery: Overcoming the obstacles. Drug Discov. Today 2013, 18, 1090–1098.
De La O, J.P.; Murtaugh, L. C. Notch and Kras in pancreatic cancer: At the crossroads of mutation, differentiation and signaling. Cell Cycle 2009, 8, 1860–1864.
Sundaram, M. V. The love-hate relationship between Ras and Notch. Genes Dev. 2005, 19, 1825–1839.
Ali, S.; Ahmad, A.; Banerjee, S.; Padhye, S.; Dominiak, K.; Schaffert, J. M.; Wang, Z. W.; Philip, P. A.; Sarkar, F. H. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res. 2010, 70, 3606–3617.
Cheng, G. Z.; Chan, J.; Wang, Q.; Zhang, W. Z.; Sun, C. D.; Wang, L. H. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007, 67, 1979–1987.
Fuchs, B. C.; Fujii, T.; Dorfman, J. D.; Goodwin, J. M.; Zhu, A. X.; Lanuti, M.; Tanabe, K. K. Epithelial-tomesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008, 68, 2391–2399.
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454.
Brabletz, T.; Jung, A.; Reu, S.; Porzner, M.; Hlubek, F.; Kunz-Schughart, L. A.; Knuechel, R.; Kirchner, T. Variable ß-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 2001, 98, 10356–10361.
Li, Y. W.; VandenBoom, T. G.; Kong, D. J.; Wang, Z. W.; Ali, S.; Philip, P. A.; Sarkar, F. H. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009, 69, 6704–6712.
Acknowledgements
The authors are grateful to Professor Chong Cheng from the University of Buffalo for the original molecular structure design of BCPV. This work has been supported by NTU-A*STAR Silicon Technologies Centre of Excellence under the program grant (No. 11235100003), start-up grant (No. M4080141.040) from Nanyang Technological University, and Grants Tier 2 MOE2010-T2-2-010 (No. M4020020.040 ARC2/11) and Tier 1 (Nos. M4010360.040 RG29/10 and M4010359.040.703012) from Ministry of Education, Singapore, and the research grant (No. MOST104-2221-E-035-078-MY2) from the “Ministry of Science and Technology”, and the grants from the National Natural Science Foundation of China (NSFC) (No. 21677102). The authors also appreciate the Precision Instrument Support Center of Feng Chia University in providing the fabrication and measurement facilities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2017_1521_MOESM1_ESM.pdf
Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine
Rights and permissions
About this article
Cite this article
Yang, C., Chan, K.K., Lin, WJ. et al. Biodegradable nanocarriers for small interfering ribonucleic acid (siRNA) co-delivery strategy increase the chemosensitivity of pancreatic cancer cells to gemcitabine. Nano Res. 10, 3049–3067 (2017). https://doi.org/10.1007/s12274-017-1521-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1521-7