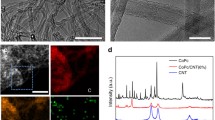
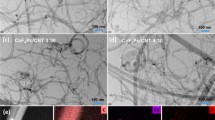
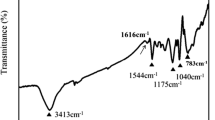
Abstract
The past decade has witnessed a rapid surge of interest in the research and development of non-precious metal-based electrocatalysts for the oxygen reduction reaction (ORR). Until now, the best catalysts in acidic electrolytes have exclusively been Fe-N-C-type materials from high-temperature pyrolysis. Despite the ORR activities of metal phthalocyanine or porphyrin macrocycles having long been known, their durability remains poor. In this work, we use these macrocycles as a basis to develop a novel organic-carbon hybrid material from in-situ polymerization of iron phthalocyanine on conductive multiwalled carbon nanotube scaffolds using a low-temperature microwave heating method. At an optimal polymerto- carbon ratio, the hybrid electrocatalyst exhibits excellent ORR activity with a positive half-wave potential (0.80 V), large mass activity (up to 18.0 A/g at 0.80 V), and a low peroxide yield (<3%). In addition, strong electronic coupling between the polymer and carbon nanotubes is believed to suppress demetallization of the macrocycles, significantly improving cycling stability in acids. Our study represents a rare example of non-precious metal-based electrocatalysts prepared without high-temperature pyrolysis, while having ORR activity in acidic media with potential for practical applications.

Similar content being viewed by others
References
Gewirth, A. A.; Thorum, M. S. Electroreduction of dioxygen for fuel-cell applications: Materials and challenges. Inorg. Chem. 2010, 49, 3557–3566.
Guo, S. J.; Zhang, S.; Sun, S. H. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2013, 52, 8526–8544.
Chen, Z. W.; Higgins, D.; Yu, A. P.; Zhang, L.; Zhang, J. J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192.
Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889.
Li, Y. G.; Zhou, W.; Wang, H. L.; Xie, L. M.; Liang, Y. Y.; Wei, F.; Idrobo, J. C.; Pennycook, S. J.; Dai, H. J. An oxygen reduction electrocatalyst based on carbon nanotubegraphene complexes. Nat. Nanotechnol. 2012, 7, 394–400.
Dai, L. M.; Xue, Y. H.; Qu, L. T.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892.
Nie, Y.; Li, L.; Wei, Z. D. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201.
Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213.
Zagal, J.; Páez, M.; Tanaka, A. A.; Dos Santos Junior, J. R., Jr.; Linkous, C. A. Electrocatalytic activity of metal phthalocyanines for oxygen reduction. J. Electroanal. Chem. 1992, 339, 13–30.
Feng, Y. J.; Alonso-Vante, N. Nonprecious metal catalysts for the molecular oxygen-reduction reaction. Phys. Status Solidi B 2008, 245, 1792–1806.
Zagal, J. H.; Griveau, S.; Silva, J. F.; Nyokong, T.; Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord. Chem. Rev. 2010, 254, 2755–2791.
Elzing, A.; Van der Putten, A.; Visscher, W.; Barendrecht, E. The cathodic reduction of oxygen at cobalt phthalocyanine: Influence of electrode preparation on electrocatalysis. J. Electroanal. Chem. Interf. Electrochem. 1986, 200, 313–322.
Van der Putten, A.; Elzing, A.; Visscher, W.; Barendrecht, E. Oxygen reduction on vacuum-deposited and adsorbed transition-metal phthalocyanine films. J. Electroanal. Chem. Interf. Electrochem. 1986, 214, 523–533.
Tanaka, A. A.; Fierro, C.; Scherson, D.; Yaeger, E. B. Electrocatalytic aspects of iron phthalocyanine and its µ-oxo derivatives dispersed on high surface area carbon. J. Phys. Chem. 1987, 91, 3799–3807.
Cao, R. G.; Thapa, R.; Kim, H.; Xu, X. D.; Gyu Kim, M.; Li, Q.; Park, N.; Liu, M. L.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076.
Jiang, Y. Y.; Lu, Y. Z.; Lv, X. Y.; Han, D. X.; Zhang, Q. X.; Niu, L.; Chen, W. Enhanced catalytic performance of Pt-free iron phthalocyanine by graphene support for efficient oxygen reduction reaction. ACS Catal. 2013, 3, 1263–1271.
Baranton, S.; Coutanceau, C.; Roux, C.; Hahn, F.; Léger, J. M. Oxygen reduction reaction in acid medium at iron phthalocyanine dispersed on high surface area carbon substrate: Tolerance to methanol, stability and kinetics. J. Electroanal. Chem. 2005, 577, 223–234.
Li, W. M.; Yu, A. P.; Higgins, D. C.; Llanos, B. G.; Chen, Z. W. Biologically inspired highly durable iron phthalocyanine catalysts for oxygen reduction reaction in polymer electrolyte membrane fuel cells. J. Am. Chem. Soc. 2010, 132, 17056–17058.
Van der Putten, A.; Elzing, A.; Visscher, W.; Barendrecht, E. Oxygen reduction on pyrolyzed carbon-supported transition metal chelates. J. Electroanal. Chem. Interf. Electrochem. 1986, 205, 233–244.
Bezerra, C. W. B.; Zhang, L.; Liu, H. S.; Lee, K. C.; Marques, A. L. B.; Marques, E. P.; Wang, H. J.; Zhang, J. J. A review of heat-treatment effects on activity and stability of PEM fuel cell catalysts for oxygen reduction reaction. J. Power Sources 2007, 173, 891–908.
Wu, G.; More, K. L.; Johnston, C. M.; Zelenay, P. Highperformance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt. Science 2011, 332, 443–447.
Masa, J.; Xia, W.; Muhler, M.; Schuhmann, W. On the role of metals in nitrogen-doped carbon electrocatalysts for oxygen reduction. Angew. Chem., Int. Ed. 2015, 54, 10102–10120.
Zhou, Y.; Wang, B.; Liu, C. H.; Han, N.; Xu, X. N.; Zhao, F. P.; Fan, J.; Li, Y. G. Polyanthraquinone-based nanostructured electrode material capable of high-performance pseudocapacitive energy storage in aprotic electrolyte. Nano Energy 2015, 15, 654–661.
Hijazi, I.; Bourgeteau, T.; Cornut, R.; Morozan, A.; Filoramo, A.; Leroy, J.; Derycke, V.; Jousselme, B.; Campidelli, S. Carbon nanotube-templated synthesis of covalent porphyrin network for oxygen reduction reaction. J. Am. Chem. Soc. 2014, 136, 6348–6354.
Tomoda, H.; Saito, S.; Ogawa, S.; Shiraishi, S. Synthesis of phthalocyanines from phthalonitrile with organic strong bases. Chem. Lett. 1980, 9, 1277–1280.
Melendres, C. A.; Cafasso, F. A. Electrochemical and in situ laser Raman spectroscopy studies on carbon-supported iron phthalocyanine electrodes. J. Electrochem. Soc. 1981, 128, 755–760.
Szybowicz, M.; Makowiecki, J. Orientation study of iron phthalocyanine (FePc) thin films deposited on silicon substrate investigated by atomic force microscopy and micro-Raman spectroscopy. J. Mater. Sci. 2012, 47, 1522–1530.
Liu, Z. Q.; Zhang, X. X.; Zhang, Y. X.; Jiang, J. Z. Theoretical investigation of the molecular, electronic structures and vibrational spectra of a series of first transition metal phthalocyanines. Spectrochim. Acta 2007, 67, 1232–1246.
Cataldo, F. Synthesis and study of electronic spectra of planar polymeric phthalocyanines. Dyes Pigm. 1997, 34, 75–85.
Liang, Y. Y.; Li, Y. G.; Wang, H. L.; Zhou, J. G.; Wang, J.; Regier, T.; Dai, H. J. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786.
Zhou, J. G.; Duchesne, P. N.; Hu, Y. F.; Wang, J.; Zhang, P.; Li, Y. G.; Regier, T.; Dai, H. J. Fe-N bonding in a carbon nanotube-graphene complex for oxygen reduction: An XAS study. Phys. Chem. Chem. Phys. 2014, 16, 15787–15791.
Cook, P. L.; Liu, X. S.; Yang, W. L.; Himpsel, F. J. X-ray absorption spectroscopy of biomimetic dye molecules for solar cells. J. Chem. Phys. 2009, 131, 194701.
Morozan, A.; Campidelli, S.; Filoramo, A.; Jousselme, B.; Palacin, S. Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 2011, 49, 4839–4847.
Li, H. J.; Xu, Z. W.; Li, K. Z.; Hou, X. H.; Cao, G. X.; Zhang, Q. L.; Cao, Z. Y. Modification of multi-walled carbon nanotubes with cobalt phthalocyanine: Effects of the templates on the assemblies. J. Mater. Chem. 2011, 21, 1181–1186.
Mamuru, S. A.; Ozoemena, K. I.; Fukuda, T.; Kobayashi, N.; Nyokong, T. Studies on the heterogeneous electron transport and oxygen reduction reaction at metal (Co, Fe) octabutylsulphonylphthalocyanines supported on multi-walled carbon nanotube modified graphite electrode. Electrochim. Acta 2010, 55, 6367–6375.
Garsany, Y.; Baturina, O. A.; Swider-Lyons, K. E.; Kocha, S. S. Experimental methods for quantifying the activity of platinum electrocatalysts for the oxygen reduction reaction. Anal. Chem. 2010, 82, 6321–6328.
Wang, Q.; Zhou, Z.-Y.; Lai, Y.-J.; You, Y.; Liu, J.-G.; Wu, X.-L.; Terefe, E.; Chen, C.; Song, L.; Rauf, M. et al. Phenylenediamine-based FeNx/C catalyst with high activity for oxygen reduction in acid medium and its active-site probing. J. Am. Chem. Soc. 2014, 136, 10882–10885.
Wang, Y.-C.; Lai, Y.-J.; Song, L.; Zhou, Z.-Y.; Liu, J.-G.; Wang, Q.; Yang, X.-D.; Chen, C.; Shi, W.; Zheng, Y.-P. et al. S-doping of an Fe/N/C ORR catalyst for polymer electrolyte membrane fuel cells with high power density. Angew. Chem., Int. Ed. 2015, 54, 9907–9910.
Bonakdarpour, A.; Dahn, T. R.; Atanasoski, R. T.; Debe, M. K.; Dahn, J. R. H2O2 release during oxygen reduction reaction on Pt nanoparticles. Electrochem. Solid-State Lett. 2008, 11, B208–B211.
Steele, B. C. H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352.
Baranton, S.; Coutanceau, C.; Garnier, E.; Léger, J. M. How does a-FePc catalysts dispersed onto high specific surface carbon support work towards oxygen reduction reaction (orr)? J. Electroanal. Chem. 2006, 590, 100–110.
Ramírez, G.; Trollund, E.; Isaacs, M.; Armijo, F.; Zagal, J.; Costamagna, J.; Aguirre, M. J. Electroreduction of molecular oxygen on poly-iron-tetraaminophthalocyanine modified electrodes. Electroanalysis 2002, 14, 540–545.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, X., Wang, B., Zhong, J. et al. Iron polyphthalocyanine sheathed multiwalled carbon nanotubes: A high-performance electrocatalyst for oxygen reduction reaction. Nano Res. 9, 1497–1506 (2016). https://doi.org/10.1007/s12274-016-1046-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-016-1046-5