Abstract
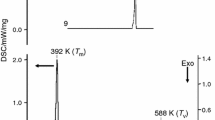
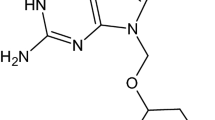
Four crystal modifications of acyclovir were isolated by recrystallization and characterized by powder X-ray diffractometry, differential scanning calorimetry, and thermogravimetric analysis. It was confirmed that Form 3 is a hydrate and Form 4 is an acetic acid solvate. The dissolution patterns of three crystal forms of acyclovir were studied in water at 37±0.5°C, 90 rpm for 120 minutes. The amount dissolved at 120 minutes was highest for Form 1, followed by Form 2 and Form 3. After storage of 25 hours at 0% RH (silica gel, 20°C) Form 3 was transformed to Form 2.
Similar content being viewed by others
References
Balfour, H. H., Drug therapy: Antiviral drugs, Review. N. Engl. J. Med., 340, 1255–1268 (1999).
Giron, D., Thermal analysis and calorimetric methods in the characterization of polymorphs and solvates. Thermochim. Acta, 248, 1–59 (1995).
Grünenberg, A., Polymorphie und thermische Analyse pharmazeutischer Wirkstoffe. Pharmazie in unserer Zeit, 26, 224–231 (1997).
Haleblian, J. K., Characterization of habits and crystalline modification of solids and their pharmaceutical applications. J. Pharm. Sci., 64, 1269–1288 (1975).
Haleblian, J. K. and McCrone, W. C., Pharmaceutical applications of polymorphism. J. Pharm. Sci., 58, 911–929 (1969).
Higuchi, W. I., Lau, P. K., Higuchi, T., and Shell, J. W., Polymorphism and drug availability, solubility relations in the methylprednisolone system. J. Pharm. Sci., 52, 150–153 (1963).
Hüttenrauch, R., Fundamentals of Pharmaceutics. Acta Pharm. Technol., 34, 1–10 (1988).
Kristl, A., Srcic, S., Vrecer, F., Sustar, B., and Vojnovic, D., Polymorphism and pseudopolymorphism: influencing the dissolution properties of the guanine derivative acyclovir. Int. J. Pharm., 139, 231–235 (1996).
Kuhnert-Brandstätter, M., Polymorphie von Arzneistoffen und ihre Bedeutung in der pharmazeutischen Technologie. Informationsdienst A.P.V., 19, 73–90 (1973).
Kuhnert-Brandstätter, M. and Lehner, G., Differentialthermoanalyse und IR-spektroskopische Untersucungen von Arzneistoffen, die als Hydrate vorliegen. Sci. Pharm., 52, 267–279 (1984).
Shefter, E. and Higuchi, T., Dissolution behavior of crystalline solvated and nonsolvated forms of some pharmaceuticals. J. Pharm. Sci., 52, 781–791 (1963).
Sohn, Y. T., Effect of crystal form on bioavailability. J. Kor. Pharm. Sci., 34, 443–452 (2004).
Zupancic, V., Ograjsek, N., Kotar-Jordan, B. and Vrecer, F., Physical characterization of pantoprazole sodium hydrates. Int. J. Pharm., 291, 59–68 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sohn, Y.T., Kim, S.H. Polymorphism and pseudopolymorphism of acyclovir. Arch. Pharm. Res. 31, 231–234 (2008). https://doi.org/10.1007/s12272-001-1146-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-001-1146-x