Abstract
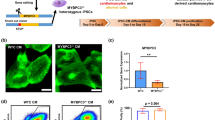
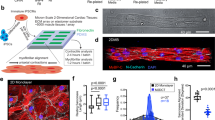
Engineered cardiac microtissues were fabricated using pluripotent stem cells with a hypertrophic cardiomyopathy associated c. 2827 C>T; p.R943x truncation variant in myosin binding protein C (MYBPC3+/−). Microtissues were mounted on iron-incorporated cantilevers, allowing manipulations of cantilever stiffness using magnets, enabling examination of how in vitro afterload affects contractility. MYPBC3+/− microtissues developed augmented force, work, and power when cultured with increased in vitro afterload when compared with isogenic controls in which the MYBPC3 mutation had been corrected (MYPBC3+/+(ed)), but weaker contractility when cultured with lower in vitro afterload. After initial tissue maturation, MYPBC3+/− CMTs exhibited increased force, work, and power in response to both acute and sustained increases of in vitro afterload. Together, these studies demonstrate that extrinsic biomechanical challenges potentiate genetically-driven intrinsic increases in contractility that may contribute to clinical disease progression in patients with HCM due to hypercontractile MYBPC3 variants.






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CMT:
-
Cardiac microtissues
- HCM:
-
Hypertrophic cardiomyopathy
- Hu-iPSC-FBs:
-
Human-induced pluripotent stem cell fibroblasts
- Hu-iPSC-CMs:
-
Human-induced pluripotent stem cell cardiomyocytes
- iPSC:
-
Induced pluripotent stem cells
- MRE:
-
Magnetorheological elastomer
- MYPBC3:
-
Myosin binding protein C
- PDMS:
-
Polydimethylsiloxane
References
Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation. 1995;92(4):785–9. https://doi.org/10.1161/01.CIR.92.4.785.
Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol. 2016;68(25):2871–86. https://doi.org/10.1016/j.jacc.2016.08.079.
Toepfer CN, Wakimoto H, Garfinkel AC, et al. Hypertrophic cardiomyopathy mutations in MYBPC3 dysregulate myosin. Sci Trans Med. 2019;11(476):eaat1199. https://doi.org/10.1126/scitranslmed.aat1199.
Davis J, Davis LC, Correll RN, et al. A tension-based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell. 2016;165(5):1147–59. https://doi.org/10.1016/j.cell.2016.04.002.
Green EM, Wakimoto H, Anderson RL, et al. Heart disease: a small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science. 2016;351(6273):617–21. https://doi.org/10.1126/science.aad3456.
Maron MS, Rowin EJ, Olivotto I, et al. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2016;67(12):1399–409. https://doi.org/10.1016/j.jacc.2016.01.023.
Maron BJ. Hypertrophic Cardiomyopathy. The Lancet. 1997;350:127–33. https://doi.org/10.1016/j.cnur.2013.09.001.
Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res. 2017;121(7):749–70. https://doi.org/10.1161/CIRCRESAHA.117.311059.
Repetti GG, Kim Y, Pereira AC, et al. Discordant clinical features of identical hypertrophic cardiomyopathy twins. Proc Natl Acad Sci U S A. 2021;118(10):3–7. https://doi.org/10.1073/pnas.2021717118.
Leonard A, Bertero A, Powers JD, et al. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J Mol Cell Cardiol. 2018;118(March):147–58. https://doi.org/10.1016/j.yjmcc.2018.03.016.
Truitt R, Mu A, Corbin EA, et al. Increased afterload augments sunitinib-induced cardiotoxicity in an engineered cardiac microtissue model. JACC Basic Transl Sci. 2018;3(2):265–76. https://doi.org/10.1016/j.jacbts.2017.12.007.
Hirt MN, Sörensen NA, Bartholdt LM, et al. Increased afterload induces pathological cardiac hypertrophy: a new in vitro model. Basic Res Cardiol. 2012;107(6):307. https://doi.org/10.1007/s00395-012-0307-z.
Rodriguez ML, Werner TR, Becker B, Eschenhagen T, Hirt MN. A magnetics-based approach for fine-tuning afterload in engineered heart tissues. ACS Biomater Sci Eng. 2019;5(7):3663–75. https://doi.org/10.1021/acsbiomaterials.8b01568.
Tang SY, Zhang X, Sun S, et al. Versatile microfluidic platforms enabled by novel magnetorheological elastomer microactuators. Adv Funct Mater. 2018;28(8):1705484. https://doi.org/10.1002/adfm.201705484.
Li Y, Li J, Li W, Du H. A state-of-the-art review on magnetorheological elastomer devices. Smart Mater Struct. 2014;23(12):123001. https://doi.org/10.1088/0964-1726/23/12/123001.
Corbin E, Vite A, Peyster EG, et al. Tunable and reversible substrate stiffness reveals dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces. 2019;11(23):20603–14. https://doi.org/10.1021/acsami.9b02446.
Seeger T, Shrestha R, Lam CK, et al. A premature termination codon mutation in MYBPC3 causes hypertrophic cardiomyopathy via chronic activation of nonsense-mediated decay. Circulation. 2019;139(6):799–811. https://doi.org/10.1161/CIRCULATIONAHA.118.034624.
Chen CY, Caporizzo MA, Bedi K, et al. Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat Med. 2018;24(8):1225–33. https://doi.org/10.1038/s41591-018-0046-2.
Legant WR, Pathak A, Yang MT, Deshpande VS, McMeeking RM, Chen CS. Microfabricated tissue gauges to measure and manipulate forces from 3D microtissues. Proc Natl Acad Sci. 2009;106(25):10097–102. https://doi.org/10.1016/0002-9394(83)90280-5.
Boudou T, Legant WR, Mu A, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A. 2012;18(9–10):910–9. https://doi.org/10.1089/ten.tea.2011.0341.
Krick BA, Vail JR, Persson BNJ, Sawyer WG. Optical in situ micro tribometer for analysis of real contact area for contact mechanics, adhesion, and sliding experiments. Tribol Lett. 2012;45(1):185–94. https://doi.org/10.1007/s11249-011-9870-y.
Vite A, Caporizzo MA, Corbin EA, et al. Extracellular stiffness induces contractile dysfunction in adult cardiomyocytes via cell-autonomous and microtubule-dependent mechanisms. Basic Res Cardiol. 2022;117(1):41. https://doi.org/10.1007/s00395-022-00952-5.
Viswanathan SK, Sanders HK, McNamara JW, et al. Hypertrophic cardiomyopathy clinical phenotype is independent of gene mutation and mutation dosage. PLoS ONE. 2017;12(11):1–19. https://doi.org/10.1371/journal.pone.0187948.
Stöhr A, Friedrich FW, Flenner F, et al. Contractile abnormalities and altered drug response in engineered heart tissue from Mybpc3-targeted knock-in mice. J Mol Cell Cardiol. 2013;63:189–98. https://doi.org/10.1016/j.yjmcc.2013.07.011.
Harris SP, Bartley CR, Hacker TA, et al. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res. 2002;90(5):594–601. https://doi.org/10.1161/01.RES.0000012222.70819.64.
Van Dijk SJ, Boontje NM, Heymans MW, et al. Preserved cross-bridge kinetics in human hypertrophic cardiomyopathy patients with MYBPC3 mutations. Pflugers Arch. 2014;466(8):1619–33. https://doi.org/10.1007/s00424-013-1391-0.
Birket MJ, Ribeiro MC, Kosmidis G, et al. Contractile defect caused by mutation in MYBPC3 revealed under conditions optimized for human PSC-cardiomyocyte function. Cell Rep. 2015;13(4):733–45. https://doi.org/10.1016/j.celrep.2015.09.025.
Ma Z, Huebsch N, Koo S, et al. Contractile deficits in engineered cardiac microtissues as a result of MYBPC3 deficiency and mechanical overload. Nat Biomed Eng. 2018;2(12):955–67. https://doi.org/10.1038/s41551-018-0280-4.
Judge D, Neamatalla H, Norris R, et al. Targeted mybpc3 knock-out mice with cardiac hypertrophy exhibit structural mitral valve abnormalities. J Cardiovasc Dev Dis. 2015;2(2):48–65. https://doi.org/10.3390/jcdd2020048.
Toepfer CN, Sharma A, Cicconet M, et al. SarcTrack. Circ Res. 2019;124(8):1172–83. https://doi.org/10.1161/CIRCRESAHA.118.314505.
Luo Q, Chen J, Zhang T, Tang X, Yu B. Retrospective analysis of clinical phenotype and prognosis of hypertrophic cardiomyopathy complicated with hypertension. Sci Rep. 2020;10(1):1–9. https://doi.org/10.1038/s41598-019-57230-z.
Zhang N, Stauffer F, Simona BR, et al. Multifunctional 3D electrode platform for real-time in situ monitoring and stimulation of cardiac tissues. Biosens Bioelectron. 2018;112(April):149–55. https://doi.org/10.1016/j.bios.2018.04.037.
Cohn R, Thakar K, Lowe A, et al. A contraction stress model of hypertrophic cardiomyopathy due to sarcomere mutations. Stem Cell Rep. 2019;12(1):71–83. https://doi.org/10.1016/j.stemcr.2018.11.015.
Funding
This research was supported by a grant from the State of Pennsylvania Department of Health, with additional support from NIH/NCATS award number TL1TR001880 to A.R. and C.E.L.; the Sidney Pestka M’61 Term Fellowship to A.R.; support from NIH T32-HL007843 to B.W.L.; American Heart Association 19CDA34770040 to C.K.L.; NIH/NHLBI grant R01 HL126527 and R01 HL130020 to J.C.W.; the Center for Engineering Mechanobiology (CEMB) under grant agreement CMMI: 15-48571 and a Provost Postdoctoral Fellowship to A.I.B.; and NIH/NHLBI grant R01-HL149891, the Leducq Foundation TNE ID#673168 and the Gordon and Llura Gund Family Fund to K.B.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human Subjects/Informed Consent/Animal Subjects Statement
While no human studies were carried out by the authors for this article, we utilized two cell lines of Hu-iPSC-CMs from the Stanford Cardiovascular Institute that were employed in the previous studies [17]. As described in Seeger et al., the protocol for induced pluripotent stem cell generation and cardiomyocyte differentiation were in accordance with the ethical standards of and approved by the Stanford Institutional Review Board (IRB) and Stem Cell Research Oversight (SCRO) Committee, and with the Helsinki Declaration of 1975, as revised in 2000. iPSC lines were obtained from patients with informed consent [17]. All procedures are followed of the responsible committee on human experimentation (institutional and national). Informed consent was obtained from all patients included in the study. No animal studies were carried out.
Competing Interests
Dr. Kenneth Margulies receives consulting fees for serving on the advisory board for Bristol-Myers-Squibb (myosin inhibitors).
Additional information
Associate Editor Paul J. R. Barton oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ramachandran, A., Livingston, C.E., Vite, A. et al. Biomechanical Impact of Pathogenic MYBPC3 Truncation Variant Revealed by Dynamically Tuning In Vitro Afterload. J. of Cardiovasc. Trans. Res. 16, 828–841 (2023). https://doi.org/10.1007/s12265-022-10348-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10348-4