Abstract
Changes in the intestinal flora and its metabolites have been associated with cardiovascular disease (CVD). Short-chain fatty acids, bile acids, and especially trimethylamine N-oxide (TMAO), an endothelial toxic factor produced by gut microbiota from phosphatidylcholine in meat, have been identified to be closely related to endothelial cell dysfunction as well as tightly affiliated with CVD, the two main types being coronary artery disease (CAD) and coronary microvascular disease (CMVD). We discuss how changes in the gut flora and the metabolite TMAO contribute to the development of CAD and CMVD. The above insight might serve as a stepping stone for novel CAD and CMVD diagnostics and therapies centered on microbiota.
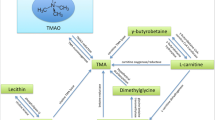
Graphical abstract


Similar content being viewed by others
Abbreviations
- CVD:
-
Cardiovascular diseases
- CAD:
-
Coronary artery diseases
- CMVD:
-
Coronary microvascular diseases
- TMAO:
-
Trimethylamine N-oxide
- ROS:
-
Reactive oxygen species
- AS:
-
Atherosclerosis
- NO:
-
Nitric oxide
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- LPS:
-
Lipopolysaccharide
- ADP:
-
Adenosine diphosphate
- CFR:
-
Coronary flow reserve
- MACEs:
-
Major adverse vascular events
- TF:
-
Tissue factor
- FMO:
-
Flavin-containing monooxygenase
- FMT:
-
Fecal microbiota transplant
- DMB:
-
3,3-Dimethyl-1-Butanol
References
Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111(25):3481–8. https://doi.org/10.1161/CIRCULATIONAHA.105.537878.
Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options. J Am Coll Cardiol. 2018;72(21):2625–41. https://doi.org/10.1016/j.jacc.2018.09.042.
Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–96. https://doi.org/10.1161/CIRCRESAHA.117.309715.
Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, …Backhed F. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci. 2011;108(Supplement_1):4592–8. https://doi.org/10.1073/pnas.1011383107.
Karlsson FH, Fåk F, Nookaew I, Tremaroli V, Fagerberg B, Petranovic D, … Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. 2012;3(1): 1245. https://doi.org/10.1038/ncomms2266.
Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, … Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nature Communications. 2017;8(1): 845. https://doi.org/10.1038/s41467-017-00900-1.
Brandsma E, Kloosterhuis NJ, Koster M, Dekker DC, Gijbels MJJ, van der Velden S, … Koonen DPY. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ Res. 2019;124(1): 94–100. https://doi.org/10.1161/CIRCRESAHA.118.313234.
Chang H-W, McNulty NP, Hibberd MC, O’Donnell D, Cheng J, Lombard V, … Gordon JI. Gut microbiome contributions to altered metabolism in a pig model of undernutrition. Proc Natl Acad Sci. 2021;118(21): e2024446118. https://doi.org/10.1073/pnas.2024446118.
Liu M, Zen K. Toll-like receptors regulate the development and progression of renal diseases. Kidney Diseases. 2021;7(1):14–23. https://doi.org/10.1159/000511947.
Liu H, Chu S, Wu Z. Loss of toll-like receptor 4 ameliorates cardiovascular dysfunction in aged mice. Immunity & Ageing. 2021;18(1):42. https://doi.org/10.1186/s12979-021-00251-y.
Molinaro A, Koh A, Wu H, Schoeler M, Faggi MI, Carreras A, … Caesar R. Hepatic expression of lipopolysaccharide-binding protein (Lbp) is induced by the gut microbiota through Myd88 and impairs glucose tolerance in mice independent of obesity. Mol Metab. 2020;37:100997. https://doi.org/10.1016/j.molmet.2020.100997.
Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8(1):36. https://doi.org/10.1186/s40168-020-00821-0.
Puteri MU, Azmi NU, Kato M, Saputri FC. PCSK9 promotes cardiovascular diseases: recent evidence about its association with platelet activation-induced myocardial infarction. Life. 2022;12(2):190. https://doi.org/10.3390/life12020190.
Chittim CL, Martínez del Campo A, Balskus EP. Gut bacterial phospholipase Ds support disease-associated metabolism by generating choline. Nat Microbiol. 2019;4(1):155–63. https://doi.org/10.1038/s41564-018-0294-4.
Phillips IR, Dolphin CT, Clair P, Hadley MR, Hutt AJ, McCombie RR, … Shephard EA. The molecular biology of the flavin-containing monooxygenases of man. Chemico-Biological Interact. 1995;96(1): 17–32. https://doi.org/10.1016/0009-2797(94)03580-2.
Al-Obaide M, Singh R, Datta P, Rewers-Felkins K, Salguero M, Al-Obaidi I, … Vasylyeva T. Gut microbiota-dependent trimethylamine-N-oxide and serum biomarkers in patients with T2DM and advanced CKD. J Clin Med. 2017;6(9): 86. https://doi.org/10.3390/jcm6090086.
Lakshmi GBVS, Yadav AK, Mehlawat N, Jalandra R, Solanki PR, Kumar A. Gut microbiota derived trimethylamine N-oxide (TMAO) detection through molecularly imprinted polymer based sensor. Sci Rep. 2021;11(1):1338. https://doi.org/10.1038/s41598-020-80122-6.
Fretts AM, Hazen SL, Jensen P, Budoff M, Sitlani CM, Wang M, … Mozaffarian D. Association of Trimethylamine N -Oxide and metabolites with mortality in older adults. JAMA Netw Open. 2022;5(5): e2213242. https://doi.org/10.1001/jamanetworkopen.2022.13242.
Ghazalpour A, Cespedes I, Bennett BJ, Allayee H. Expanding role of gut microbiota in lipid metabolism. Curr Opin Lipidol. 2016;27(2):141–7. https://doi.org/10.1097/MOL.0000000000000278.
Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, … Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. https://doi.org/10.1016/j.cell.2016.02.011.
Catar R, Chen L, Zhao H, Wu D, Kamhieh-Milz J, Lücht C, … Witowski J. Native and oxidized low-density lipoproteins increase the expression of the LDL receptor and the LOX-1 receptor, respectively, in arterial endothelial cells. Cells. 2022;11(2):204. https://doi.org/10.3390/cells11020204.
Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, Li J. Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway. Biomed Pharmacother. 2018;97:941–7. https://doi.org/10.1016/j.biopha.2017.11.016.
Cheng X, Qiu X, Liu Y, Yuan C, Yang X. Trimethylamine N-oxide promotes tissue factor expression and activity in vascular endothelial cells: a new link between trimethylamine N-oxide and atherosclerotic thrombosis. Thromb Res. 2019;177:110–6. https://doi.org/10.1016/j.thromres.2019.02.028.
Wu P, Chen J, Chen J, Tao J, Wu S, Xu G, … Yin W. Trimethylamine N‐oxide promotes apoE −/− mice atherosclerosis by inducing vascular endothelial cell pyroptosis via the SDHB/ROS pathway. J Cell Physiol. 2020;235(10):6582–6591. https://doi.org/10.1002/jcp.29518.
Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, … Yusuf S. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART Study. Circulation. 2008;118(19):1929–1937. https://doi.org/10.1161/CIRCULATIONAHA.107.738716.
Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjønneland A, … Linseisen J. Meat consumption and mortality - results from the European prospective investigation into cancer and nutrition. BMC Med. 2013;11(1):63. https://doi.org/10.1186/1741-7015-11-63.
Simsek EC, Sari C, Kucukokur M, Ekmekci C, Colak A, Ozdogan O. Endothelial dysfunction in patients with myocardial ischemia or infarction and nonobstructive coronary arteries. J Clin Ultrasound. 2021;49(4):334–40. https://doi.org/10.1002/jcu.22902.
Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, … Kaufmann PA. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography. J Am Coll Cardiol. 2009; 54(2):150–156. https://doi.org/10.1016/j.jacc.2009.02.069.
Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014;35(17):1101–11. https://doi.org/10.1093/eurheartj/eht513.
Shin D, Dai N, Lee SH, Choi KH, Lefieux A, Molony D, … Lee JM. Physiological distribution and local severity of coronary artery disease and outcomes after percutaneous coronary intervention. JACC Cardiovasc Interv. 2021;14(16):1771–1785. https://doi.org/10.1016/j.jcin.2021.06.013.
Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular angina. J Am Coll Cardiol. 2018;72(21):2642–62. https://doi.org/10.1016/j.jacc.2018.07.106.
Brunt VE, Gioscia-Ryan RA, Casso AG, Van Dongen NS, Ziemba BP, Sapinsley ZJ, … Seals DR. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76(1):101–112. https://doi.org/10.1161/HYPERTENSIONAHA.120.14759.
Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481(1–2):63–70. https://doi.org/10.1016/j.bbrc.2016.11.017.
Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, … Sopko G. Insights from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2006;47(3):S21–S29. https://doi.org/10.1016/j.jacc.2004.12.084.
Tan C, Wang H, Gao X, Xu R, Zeng X, Cui Z, … Yin J. Dynamic changes and prognostic value of gut microbiota-dependent trimethylamine-N-oxide in acute ischemic stroke. Front Neurol. 2020;11:29. https://doi.org/10.3389/fneur.2020.00029.
Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens J-F, … Delzenne NM. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67(2):271–283. https://doi.org/10.1136/gutjnl-2016-313316.
Rashid SK, Khodja NI, Auger C, Alhosin M, Boehm N, Oswald-Mammosser M, Schini-Kerth VB. Probiotics (VSL#3) prevent endothelial dysfunction in rats with portal hypertension: role of the angiotensin system. PLoS ONE. 2014;9(5): e97458. https://doi.org/10.1371/journal.pone.0097458.
Gómez-Guzmán M, Toral M, Romero M, Jiménez R, Galindo P, Sánchez M, … Duarte J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res. 2015;59(11):2326–2336. https://doi.org/10.1002/mnfr.201500290.
Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, … Duarte J. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci. 2014;127(1):33–45. https://doi.org/10.1042/CS20130339.
Lu Q, Guo Y, Yang G, Cui L, Wu Z, Zeng X, … Cai Z. Structure and anti-inflammation potential of lipoteichoic acids isolated from Lactobacillus strains. Foods. 2022;11(11):1610. https://doi.org/10.3390/foods11111610.
Yan R, Wang K, Wang Q, Jiang H, Lu Y, Chen X, … Lv L. Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders. Microb Biotechnol. 2022;15(1):247–261. https://doi.org/10.1111/1751-7915.13750.
Malik M, Suboc TM, Tyagi S, Salzman N, Wang J, Ying R, … Widlansky ME. Lactobacillus plantarum 299v Supplementation improves vascular endothelial function and reduces inflammatory biomarkers in men with stable coronary artery disease. Circ Res. 2018;123(9):1091–1102. https://doi.org/10.1161/CIRCRESAHA.118.313565.
Aroniadis OC, Brandt LJ. Fecal microbiota transplantation: past, present and future. Curr Opin Gastroenterol. 2013;29(1):79–84. https://doi.org/10.1097/MOG.0b013e32835a4b3e.
Tariq R, Pardi DS, Bartlett MG, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. 2019;68(8):1351–8. https://doi.org/10.1093/cid/ciy721.
Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, … Nieuwdorp M. Effect of vegan fecal microbiota transplantation on carnitine‐ and choline‐derived trimethylamine‐N‐oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018;7(7). https://doi.org/10.1161/JAHA.117.008342.
Santos-Marcos JA, Perez-Jimenez F, Camargo A. The role of diet and intestinal microbiota in the development of metabolic syndrome. J Nutr Biochem. 2019;70:1–27. https://doi.org/10.1016/j.jnutbio.2019.03.017.
Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci. 2012;109(52):21307–12. https://doi.org/10.1073/pnas.1215689109.
Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, … Hazen SL. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. https://doi.org/10.1016/j.cell.2015.11.055.
Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–70. https://doi.org/10.1161/CIRCRESAHA.120.316242.
Cho CE, Caudill MA. Trimethylamine- N -oxide: friend, foe, or simply caught in the cross-fire? Trends Endocrinol Metab. 2017;28(2):121–30. https://doi.org/10.1016/j.tem.2016.10.005.
Witkowski M, Witkowski M, Friebel J, Buffa JA, Li XS, Wang Z, … Hazen SL. Vascular endothelial tissue factor contributes to trimethylamine N-oxide-enhanced arterial thrombosis. Cardiovasc Res. 2021;cvab263. https://doi.org/10.1093/cvr/cvab263.
Romano KA, Vivas EI, Amador-Noguez D, Rey FE. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6(2):e02481-14. https://doi.org/10.1128/mBio.02481-14.
Pathak P, Helsley RN, Brown AL, Buffa JA, Choucair I, Nemet I, … Brown JM. Small molecule inhibition of gut microbial choline trimethylamine lyase activity alters host cholesterol and bile acid metabolism. Am J Physiol-Heart Circ Physiol. 2020;318(6):H1474–H1486. https://doi.org/10.1152/ajpheart.00584.2019.
Simó C, Fornari T, García-Risco MR, Peña-Cearra A, Abecia L, Anguita J, … García-Cañas V. Resazurin-based high-throughput screening method for the discovery of dietary phytochemicals to target microbial transformation of l-carnitine into trimethylamine, a gut metabolite associated with cardiovascular disease. Food Func. 2022;13(10): 5640–5653. https://doi.org/10.1039/D2FO00103A.
Chen M, Yi L, Zhang Y, Zhou X, Ran L, Yang J, … Mi M. Resveratrol attenuates trimethylamine- N -oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. 2016;7(2): e02210–15. https://doi.org/10.1128/mBio.02210-15.
Gabr M, Świderek K. Discovery of a histidine-based scaffold as an inhibitor of gut microbial choline trimethylamine-lyase. ChemMedChem. 2020;15(23):2273–9. https://doi.org/10.1002/cmdc.202000571.
Gabr MT, Machalz D, Pach S, Wolber G. A benzoxazole derivative as an inhibitor of anaerobic choline metabolism by human gut microbiota. RSC Med Chem. 2020;11(12):1402–12. https://doi.org/10.1039/D0MD00218F.
Gabr MT, Deganutti G, Reynolds CA. Peptidomimetic-based approach toward inhibitors of microbial trimethylamine lyases. Chem Biol Drug Des. 2021;97(2):231–6. https://doi.org/10.1111/cbdd.13775.
Anwar S, Bhandari U, Panda BP, Dubey K, Khan W, Ahmad S. Trigonelline inhibits intestinal microbial metabolism of choline and its associated cardiovascular risk. J Pharm Biomed Anal. 2018;159:100–12. https://doi.org/10.1016/j.jpba.2018.06.027.
Orman M, Bodea S, Funk MA, Campo AM, Bollenbach M, Drennan CL, Balskus EP. Structure-guided identification of a small molecule that inhibits anaerobic choline metabolism by human gut bacteria. J Am Chem Soc. 2019;141(1):33–7. https://doi.org/10.1021/jacs.8b04883.
Funding
This research was funded by Clinical Research Plan of SHDC, grant number 2020CR4065; National Natural Science Foundation of China, grant number 82071956.
Author information
Authors and Affiliations
Contributions
F.Y. and H.Z. had the idea for the article; Y.H. performed the literature search and initial writing; H.Z. and X.F. critically revised the work. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Informed Consent
No human studies were carried out by the authors for this article.
Additional information
Associate Editor Yihua Bei oversaw the review of this article
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Y., Zhang, H., Fan, X. et al. The Role of Gut Microbiota and Trimethylamine N-oxide in Cardiovascular Diseases. J. of Cardiovasc. Trans. Res. 16, 581–589 (2023). https://doi.org/10.1007/s12265-022-10330-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10330-0