Abstract
Several prior studies have highlighted the promise of mesenchymal stem cells (MSCs) as tools for treating myocardial infarction (MI) patients. While MSCs were initially thought to mediate post-MI repair through differentiation and replacement of injured cells, they are now thought to function by releasing exosomes carrying important cargos which can prevent apoptosis and facilitate revascularization in the context of MI. Herein, we comprehensively survey prior preclinical studies examining MSC-derived exosomes (MSC-Exos) utility for the repair of MI-related tissue injury. In total, 24 relevant studies were identified in the PubMed, Web of Science, Embase, and Cochrane Library databases as per the PRISMA guidelines. In most studies, exosome-treated rodents exhibited improved cardiac function and angiogenesis together with decreased apoptotic cell death. MSC-Exos thus offer beneficial therapeutic efficacy when treating MI injury. However, further work will be necessary to standardize experimental preclinical models and to validate these results.
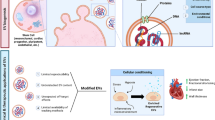
Graphical abstract
This systematic review provides a comprehensive overview of previous preclinical studies on the utility of exosomes derived from mesenchymal stem cells (MSCs) in the repair of myocardial infarction (MI) injury.


Similar content being viewed by others
Abbreviations
- ATV:
-
Atorvastatin
- CSCs:
-
Cardiac stem cells
- EVs:
-
Extracellular vesicles
- HF:
-
Heart failure
- LAD:
-
Left anterior descending artery
- lncRNAs:
-
Long non-coding RNAs
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular fractional shortening
- LVESD:
-
Left ventricular end-systolic diameter
- LVEDD:
-
Left ventricular end-diastolic diameter
- Mecp2:
-
Methyl CpG binding protein 2
- MI:
-
Myocardial infarction
- MIF:
-
Macrophage migration inhibitory factor
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MSCs:
-
Mesenchymal stem cells
- MSC-Exos:
-
MSC-derived exosomes
- vWF:
-
von Willebrand factor
References
McCarroll, C. S., He, W., Foote, K., Bradley, A., McGlynn, K., Vidler, F., et al. (2018). Runx1 deficiency protects against adverse cardiac remodeling after myocardial infarction. Circulation, 137(1), 57–70. https://doi.org/10.1161/circulationaha.117.028911
Spiliopoulos, S., Koerfer, R., & Tenderich, G. (2016). Acute myocardial infarction complicated by cardiogenic shock: Results of primary percutaneous coronary interventions are insufficient. European Journal of Cardio-Thoracic Surgery, 49(4), 1298. https://doi.org/10.1093/ejcts/ezv331
Shafei, A. E. S., Ali, M. A., Ghanem, H. G., Shehata, A. I., Abdelgawad, A. A., Handal, H. R., et al. (2017). Mesenchymal stem cell therapy: A promising cell-based therapy for treatment of myocardial infarction. The Journal of Gene Medicine, 19(12), e2995. https://doi.org/10.1002/jgm.2995
Bao, L., Meng, Q., Li, Y., Deng, S., Yu, Z., Liu, Z., et al. (2017). C-Kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. Journal of Cardiac Failure, 23(5), 403–415. https://doi.org/10.1016/j.cardfail.2017.03.002
Dakhlallah, D., Zhang, J., Yu, L., Marsh, C. B., Angelos, M. G., & Khan, M. (2015). MicroRNA-133a engineered mesenchymal stem cells augment cardiac function and cell survival in the infarct heart. Journal of Cardiovascular Pharmacology, 65(3), 241–251. https://doi.org/10.1097/fjc.0000000000000183
Wen, Z., Zheng, S., Zhou, C., Yuan, W., Wang, J., & Wang, T. (2012). Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: MicroRNAs as novel regulators. Journal of Cellular and Molecular Medicine, 16(4), 657–671. https://doi.org/10.1111/j.1582-4934.2011.01471.x
Song, M., Heo, J., Chun, J. Y., Bae, H. S., Kang, J. W., Kang, H., et al. (2014). The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells and Development, 23(6), 654–663. https://doi.org/10.1089/scd.2013.0277
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., & Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplantation, 23(9), 1045–1059. https://doi.org/10.3727/096368913x667709
Bogatcheva, N. V., & Coleman, M. E. (2019). Conditioned medium of mesenchymal stromal cells: A new class of therapeutics. Biochemistry, 84(11), 1375–1389. https://doi.org/10.1134/s0006297919110129
Lelek, J., & Zuba-Surma, E. K. (2020). Perspectives for future use of extracellular vesicles from umbilical cord- and adipose tissue-derived mesenchymal stem/stromal cells in regenerative therapies-synthetic review. International Journal of Molecular Sciences, 21(3), 799. https://doi.org/10.3390/ijms21030799
Tsiapalis, D., & O’Driscoll, L. (2020). Mesenchymal stem cell derived extracellular vesicles for tissue engineering and regenerative medicine applications. Cells, 9(4), 991. https://doi.org/10.3390/cells9040991
Nazarenko, I. (2020). Extracellular vesicles: Recent developments in technology and perspectives for cancer liquid biopsy. Recent Results in Cancer Research, 215, 319–344. https://doi.org/10.1007/978-3-030-26439-0_17
Mignot, G., Roux, S., Thery, C., Ségura, E., & Zitvogel, L. (2006). Prospects for exosomes in immunotherapy of cancer. Journal of Cellular and Molecular Medicine, 10(2), 376–388. https://doi.org/10.1111/j.1582-4934.2006.tb00406.x
Chaput, N., Flament, C., Viaud, S., Taieb, J., Roux, S., Spatz, A., et al. (2006). Dendritic cell derived-exosomes: Biology and clinical implementations. Journal of Leukocyte Biology, 80(3), 471–478. https://doi.org/10.1189/jlb.0206094
Jin, J., & Menon, R. (2018). Placental exosomes: A proxy to understand pregnancy complications. American Journal of Reproductive Immunology, 79(5), e12788. https://doi.org/10.1111/aji.12788
Hoeeg, C., Frljak, S., Qayyum, A. A., Vrtovec, B., Kastrup, J., Ekblond, A., et al. (2020). Efficacy and mode of action of mesenchymal stem cells in non-ischemic dilated cardiomyopathy: A systematic review. Biomedicines, 8(12), 570. https://doi.org/10.3390/biomedicines8120570
Harrell, C. R., Jovicic, N., Djonov, V., Arsenijevic, N., & Volarevic, V. (2019). Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells, 8(12), 1605. https://doi.org/10.3390/cells8121605
Tan, S. J. O., Floriano, J. F., Nicastro, L., Emanueli, C., & Catapano, F. (2020). Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules, 10(5), 707. https://doi.org/10.3390/biom10050707
Harrell, C. R., Jovicic, N., Djonov, V., & Volarevic, V. (2020). Therapeutic use of mesenchymal stem cell-derived exosomes: From basic science to clinics. Pharmaceutics, 12(5), 474. https://doi.org/10.3390/pharmaceutics12050474
Mokhtari, B., Aboutaleb, N., Nazarinia, D., Nikougoftar, M., Razavi Tousi, S. M. T., Molazem, M., et al. (2020). Comparison of the effects of intramyocardial and intravenous injections of human mesenchymal stem cells on cardiac regeneration after heart failure. Iranian Journal of Basic Medical Sciences, 23, 879. https://doi.org/10.22038/ijbms.2020.40886.9660
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., & Langendam, M. W. (2014). SYRCLE’s risk of bias tool for animal studies. BMC Medical Research Methodology, 14, 43. https://doi.org/10.1186/1471-2288-14-43
Feng, Y., Huang, W., Wani, M., Yu, X., & Ashraf, M. (2014). Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE, 9(2), e88685. https://doi.org/10.1371/journal.pone.0088685
Kang, K., Ma, R., Cai, W., Huang, W., Paul, C., Liang, J., et al. (2015). Exosomes secreted from CXCR4 overexpressing mesenchymal stem cells promote cardioprotection via Akt signaling pathway following myocardial infarction. Stem Cells International, 2015, 659890. https://doi.org/10.1155/2015/659890
Yu, B., Kim, H. W., Gong, M., Wang, J., Millard, R. W., Wang, Y., et al. (2015). Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. International Journal of Cardiology, 182, 349–360. https://doi.org/10.1016/j.ijcard.2014.12.043
Teng, X., Chen, L., Chen, W., Yang, J., Yang, Z., & Shen, Z. (2015). Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cellular Physiology and Biochemistry, 37(6), 2415–2424. https://doi.org/10.1159/000438594
Zhang, Z., Yang, J., Yan, W., Li, Y., Shen, Z., & Asahara, T. (2016). Pretreatment of cardiac stem cells with exosomes derived from mesenchymal stem cells enhances myocardial repair. Journal of the American Heart Association, 5(1), e002856. https://doi.org/10.1161/jaha.115.002856
Shao, L., Zhang, Y., Lan, B., Wang, J., Zhang, Z., Zhang, L., et al. (2017). MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Research International, 2017, 4150705. https://doi.org/10.1155/2017/4150705
He, J. G., Li, H. R., Han, J. X., Li, B. B., Yan, D., Li, H. Y., et al. (2018). GATA-4-expressing mouse bone marrow mesenchymal stem cells improve cardiac function after myocardial infarction via secreted exosomes. Scientific Reports, 8(1), 9047. https://doi.org/10.1038/s41598-018-27435-9
Zhu, L. P., Tian, T., Wang, J. Y., He, J. N., Chen, T., Pan, M., et al. (2018). Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics, 8(22), 6163–6177. https://doi.org/10.7150/thno.28021
Ma, T., Chen, Y., Chen, Y., Meng, Q., Sun, J., Shao, L., et al. (2018). MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells International, 2018, 3290372. https://doi.org/10.1155/2018/3290372
Zhu, J., Lu, K., Zhang, N., Zhao, Y., Ma, Q., Shen, J., et al. (2018). Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artificial Cells, Nanomedicine, and Biotechnology, 46(8), 1659–1670. https://doi.org/10.1080/21691401.2017.1388249
Xiao, C., Wang, K., Xu, Y., Hu, H., Zhang, N., Wang, Y., et al. (2018). Transplanted mesenchymal stem cells reduce autophagic flux in infarcted hearts via the exosomal transfer of miR-125b. Circulation Research, 123(5), 564–578. https://doi.org/10.1161/circresaha.118.312758
Zou, L., Ma, X., Lin, S., Wu, B., Chen, Y., & Peng, C. (2019). Bone marrow mesenchymal stem cell-derived exosomes protect against myocardial infarction by promoting autophagy. Experimental and Therapeutic Medicine, 18(4), 2574–2582. https://doi.org/10.3892/etm.2019.7874
Huang, P., Wang, L., Li, Q., Xu, J., Xu, J., Xiong, Y., et al. (2019). Combinatorial treatment of acute myocardial infarction using stem cells and their derived exosomes resulted in improved heart performance. Stem Cell Research & Therapy, 10(1), 300. https://doi.org/10.1186/s13287-019-1353-3
Li, Y., Yang, R., Guo, B., Zhang, H., Zhang, H., Liu, S., et al. (2019). Exosomal miR-301 derived from mesenchymal stem cells protects myocardial infarction by inhibiting myocardial autophagy. Biochemical and Biophysical Research Communications, 514(1), 323–328. https://doi.org/10.1016/j.bbrc.2019.04.138
Xu, R., Zhang, F., Chai, R., Zhou, W., Hu, M., Liu, B., et al. (2019). Exosomes derived from pro-inflammatory bone marrow-derived mesenchymal stem cells reduce inflammation and myocardial injury via mediating macrophage polarization. Journal of Cellular and Molecular Medicine, 23(11), 7617–7631. https://doi.org/10.1111/jcmm.14635
Zhang, C. S., Shao, K., Liu, C. W., Li, C. J., & Yu, B. T. (2019). Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. European Review for Medical and Pharmacological Sciences, 23(15), 6691–6699. https://doi.org/10.26355/eurrev_201908_18560
Huang, P., Wang, L., Li, Q., Tian, X., Xu, J., Xu, J., et al. (2020). Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovascular Research, 116(2), 353–367. https://doi.org/10.1093/cvr/cvz139
Li, Y., Zhou, J., Zhang, O., Wu, X., Guan, X., Xue, Y., et al. (2020). Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. International Immunopharmacology, 80, 106156. https://doi.org/10.1016/j.intimp.2019.106156
Sun, L., Zhu, W., Zhao, P., Zhang, J., Lu, Y., Zhu, Y., et al. (2020). Down-regulated exosomal MicroRNA-221 - 3p derived from senescent mesenchymal stem cells impairs heart repair. Frontiers in Cell and Developmental Biology, 8, 263. https://doi.org/10.3389/fcell.2020.00263
Liu, X., Li, X., Zhu, W., Zhang, Y., Hong, Y., Liang, X., et al. (2020). Exosomes from mesenchymal stem cells overexpressing MIF enhance myocardial repair. Journal of Cellular Physiology, 235(11), 8010–8022. https://doi.org/10.1002/jcp.29456
Sun, J., Shen, H., Shao, L., Teng, X., Chen, Y., Liu, X., et al. (2020). HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Research & Therapy, 11(1), 373. https://doi.org/10.1186/s13287-020-01881-7
Cheng, H., Chang, S., Xu, R., Chen, L., Song, X., Wu, J., et al. (2020). Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Research & Therapy, 11(1), 224. https://doi.org/10.1186/s13287-020-01737-0
Fu, D. L., Jiang, H., Li, C. Y., Gao, T., Liu, M. R., & Li, H. W. (2020). MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. European Review for Medical and Pharmacological Sciences, 24(19), 10107–10117. https://doi.org/10.26355/eurrev_202010_23230
Wang, S., Li, L., Liu, T., Jiang, W., & Hu, X. (2020). miR-19a/19b-loaded exosomes in combination with mesenchymal stem cell transplantation in a preclinical model of myocardial infarction. Regenerative Medicine, 15(6), 1749–1759. https://doi.org/10.2217/rme-2019-0136
Cheng, W., Wang, L., Yang, T., Wu, A., Wang, B., Li, T., et al. (2020). Qiliqiangxin capsules optimize cardiac metabolism flexibility in rats with heart failure after myocardial infarction. Frontiers in Physiology, 11, 805. https://doi.org/10.3389/fphys.2020.00805
He, X., Yao, M. W., Zhu, M., Liang, D. L., Guo, W., Yang, Y., et al. (2018). Metformin induces apoptosis in mesenchymal stromal cells and dampens their therapeutic efficacy in infarcted myocardium. Stem Cell Research & Therapy, 9(1), 306. https://doi.org/10.1186/s13287-018-1057-0
Yu, W., Sun, S., Xu, H., Li, C., Ren, J., & Zhang, Y. (2020). TBC1D15/RAB7-regulated mitochondria-lysosome interaction confers cardioprotection against acute myocardial infarction-induced cardiac injury. Theranostics, 10(24), 11244–11263. https://doi.org/10.7150/thno.46883
Zhang, H., Yin, Y., Liu, Y., Zou, G., Huang, H., Qian, P., et al. (2020). Necroptosis mediated by impaired autophagy flux contributes to adverse ventricular remodeling after myocardial infarction. Biochemical Pharmacology, 175, 113915. https://doi.org/10.1016/j.bcp.2020.113915
Walker, B. W., Lara, R. P., Yu, C. H., Sani, E. S., Kimball, W., Joyce, S., et al. (2019). Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials, 207, 89–101. https://doi.org/10.1016/j.biomaterials.2019.03.015
Won, Y. W., Bull, D. A., & Kim, S. W. (2014). Functional polymers of gene delivery for treatment of myocardial infarct. Journal of Controlled Release, 195, 110–119. https://doi.org/10.1016/j.jconrel.2014.07.041
Zeng, Y., Li, J., Wang, H. X., Guo, S. B., Yang, H., Zeng, X. J., et al. (2013). Transcriptional effects of E3 ligase atrogin-1/MAFbx on apoptosis, hypertrophy and inflammation in neonatal rat cardiomyocytes. PLoS ONE, 8(1), e53831. https://doi.org/10.1371/journal.pone.0053831
Kim, S. H., Jeong, J. H., Ou, M., Yockman, J. W., Kim, S. W., & Bull, D. A. (2008). Cardiomyocyte-targeted siRNA delivery by prostaglandin E(2)-Fas siRNA polyplexes formulated with reducible poly(amido amine) for preventing cardiomyocyte apoptosis. Biomaterials, 29(33), 4439–4446. https://doi.org/10.1016/j.biomaterials.2008.07.047
Huang, S., & Frangogiannis, N. G. (2018). Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. British Journal of Pharmacology, 175(9), 1377–1400. https://doi.org/10.1111/bph.14155
Adrover, J. M., Del Fresno, C., Crainiciuc, G., Cuartero, M. I., Casanova-Acebes, M., Weiss, L. A., et al. (2019). A neutrophil timer coordinates immune defense and vascular protection. Immunity, 50(2), 390-402.e310. https://doi.org/10.1016/j.immuni.2019.01.002
Gast, M., Rauch, B. H., Haghikia, A., Nakagawa, S., Haas, J., Stroux, A., et al. (2019). Long noncoding RNA NEAT1 modulates immune cell functions and is suppressed in early onset myocardial infarction patients. Cardiovascular Research, 115(13), 1886–1906. https://doi.org/10.1093/cvr/cvz085
Sahoo, S., & Losordo, D. W. (2014). Exosomes and cardiac repair after myocardial infarction. Circulation Research, 114(2), 333–344. https://doi.org/10.1161/circresaha.114.300639
Yu, H., Lu, K., Zhu, J., & Wang, J. (2017). Stem cell therapy for ischemic heart diseases. British Medical Bulletin, 121(1), 135–154. https://doi.org/10.1093/bmb/ldw059
Bernstock, J. D., Peruzzotti-Jametti, L., Ye, D., Gessler, F. A., Maric, D., Vicario, N., et al. (2017). Neural stem cell transplantation in ischemic stroke: A role for preconditioning and cellular engineering. Journal of Cerebral Blood Flow and Metabolism, 37(7), 2314–2319. https://doi.org/10.1177/0271678x17700432
Maffioletti, S. M., Noviello, M., English, K., & Tedesco, F. S. (2014). Stem cell transplantation for muscular dystrophy: The challenge of immune response. BioMed Research International, 2014, 964010. https://doi.org/10.1155/2014/964010
Duran, J. M., Makarewich, C. A., Sharp, T. E., Starosta, T., Zhu, F., Hoffman, N. E., et al. (2013). Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circulation Research, 113(5), 539–552. https://doi.org/10.1161/circresaha.113.301202
Vizoso, F. J., Eiro, N., Cid, S., Schneider, J., & Perez-Fernandez, R. (2017). Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. International Journal of Molecular Sciences, 18(9), 1852. https://doi.org/10.3390/ijms18091852
Bacakova, L., Zarubova, J., Travnickova, M., Musilkova, J., Pajorova, J., Slepicka, P., et al. (2018). Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - A review. Biotechnology Advances, 36(4), 1111–1126. https://doi.org/10.1016/j.biotechadv.2018.03.011
Nesselmann, C., Ma, N., Bieback, K., Wagner, W., Ho, A., Konttinen, Y. T., et al. (2008). Mesenchymal stem cells and cardiac repair. Journal of Cellular and Molecular Medicine, 12(5b), 1795–1810. https://doi.org/10.1111/j.1582-4934.2008.00457.x
Khasawneh, R. R., Abu-El-Rub, E., Serhan, A. O., Serhan, B. O., & Abu-El-Rub, H. (2019). Cross talk between 26S proteasome and mitochondria in human mesenchymal stem cells’ ability to survive under hypoxia stress. Journal of Physiological Sciences, 69(6), 1005–1017. https://doi.org/10.1007/s12576-019-00720-6
Mead, B., & Tomarev, S. (2017). Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Translational Medicine, 6(4), 1273–1285. https://doi.org/10.1002/sctm.16-0428
Li, N., Rochette, L., Wu, Y., & Rosenblatt-Velin, N. (2019). New insights into the role of exosomes in the heart after myocardial infarction. Journal of Cardiovascular Translational Research, 12(1), 18–27. https://doi.org/10.1007/s12265-018-9831-z
Katare, R., Riu, F., Mitchell, K., Gubernator, M., Campagnolo, P., Cui, Y., et al. (2011). Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circulation Research, 109(8), 894–906. https://doi.org/10.1161/circresaha.111.251546
Sun, L., Zhang, Y., Zhang, J., Wang, J., & Xing, S. (2020). Atorvastatin improves the proliferation and migration of endothelial progenitor cells via the miR-221/VEGFA axis. Bioscience Reports, 40(11), BSR20193053. https://doi.org/10.1042/BSR20193053
Sayed, D., & Abdellatif, M. (2011). MicroRNAs in development and disease. Physiological Reviews, 91(3), 827–887. https://doi.org/10.1152/physrev.00006.2010
Kir, D., Schnettler, E., Modi, S., & Ramakrishnan, S. (2018). Regulation of angiogenesis by microRNAs in cardiovascular diseases. Angiogenesis, 21(4), 699–710. https://doi.org/10.1007/s10456-018-9632-7
Coskunpinar, E., Cakmak, H. A., Kalkan, A. K., Tiryakioglu, N. O., Erturk, M., & Ongen, Z. (2016). Circulating miR-221-3p as a novel marker for early prediction of acute myocardial infarction. Gene, 591(1), 90–96. https://doi.org/10.1016/j.gene.2016.06.059
Täubel, J., Hauke, W., Rump, S., Viereck, J., Batkai, S., Poetzsch, J., et al. (2021). Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. European Heart Journal, 42(2), 178–188. https://doi.org/10.1093/eurheartj/ehaa898
Zhang, Y., Zhu, W., He, H., Fan, B., Deng, R., Hong, Y., et al. (2019). Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging, 11(24), 12641–12660. https://doi.org/10.18632/aging.102592
Gidlöf, O., van der Brug, M., Ohman, J., Gilje, P., Olde, B., Wahlestedt, C., et al. (2013). Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood, 121(19), 3908–3917. https://doi.org/10.1182/blood-2012-10-461798 s3901–3926.
Silva, D., Carneiro, F. D., Almeida, K. C., & Fernandes-Santos, C. (2018). Role of miRNAs on the pathophysiology of cardiovascular diseases. Arquivos Brasileiros de Cardiologia, 111(5), 738–746. https://doi.org/10.5935/abc.20180215
Li, L., Li, S., Wu, M., Chi, C., Hu, D., Cui, Y., et al. (2019). Early diagnostic value of circulating microRNAs in patients with suspected acute myocardial infarction. Journal of Cellular Physiology, 234(8), 13649–13658. https://doi.org/10.1002/jcp.28045
Dai, S., Wei, D., Wu, Z., Zhou, X., Wei, X., Huang, H., et al. (2008). Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Molecular Therapy, 16(4), 782–790. https://doi.org/10.1038/mt.2008.1
Dougherty, J. A., Mergaye, M., Kumar, N., Chen, C. A., Angelos, M. G., & Khan, M. (2017). Potential Role of Exosomes in Mending a Broken Heart: Nanoshuttles Propelling Future Clinical Therapeutics Forward. Stem Cells International, 2017, 5785436. https://doi.org/10.1155/2017/5785436
Kishore, R., & Khan, M. (2016). More than tiny sacks: Stem cell exosomes as cell-free modality for cardiac repair. Circulation Research, 118(2), 330–343. https://doi.org/10.1161/circresaha.115.307654
Faggion, C. M., Jr., Diaz, K. T., Aranda, L., Gabel, F., Listl, S., & Alarcón, M. A. (2017). The risk of bias of animal experiments in implant dentistry: A methodological study. Clinical Oral Implants Research, 28(7), e39–e45. https://doi.org/10.1111/clr.12852
du Sert, N. P., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., et al. (2020). The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biology, 18(7), e3000410. https://doi.org/10.1371/journal.pbio.3000410
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81973787) and the Postdoctoral Research Foundation of China (2019M660574).
Author information
Authors and Affiliations
Contributions
H.M. and W.T.C. have contributed equally to this work. Theme and design of the research: H.M.; article retrieval: H.M. and L.W.; data extraction: S.Q.C., Y.T., Z.W.L., Y.L., and W.T.C.; verification of data: H.M.; writing of the manuscript: H.M.; critical revision of the manuscript for intellectual content: W.T.C. and M.J.Z.; obtaining funding: M.J.Z. and H.M.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of Interest
The authors declare no competing interests.
Additional information
Communicated by Associate Editor Junjie Xiao oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, H., Cheng, W., Wang, L. et al. Mesenchymal Stem Cell Exosomes in the Treatment of Myocardial Infarction: a Systematic Review of Preclinical In Vivo Studies. J. of Cardiovasc. Trans. Res. 15, 317–339 (2022). https://doi.org/10.1007/s12265-021-10168-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-021-10168-y