Abstract
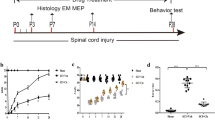
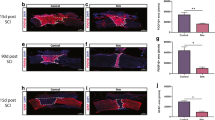
Epidural spinal cord stimulation (ESCS) markedly improves motor and sensory function after spinal cord injury (SCI), but the underlying mechanisms are unclear. Here, we investigated whether ESCS affects oligodendrocyte differentiation and its cellular and molecular mechanisms in rats with SCI. ESCS improved hindlimb motor function at 7 days, 14 days, 21 days, and 28 days after SCI. ESCS also significantly increased the myelinated area at 28 days, and reduced the number of apoptotic cells in the spinal white matter at 7 days. SCI decreased the expression of 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase, an oligodendrocyte marker) at 7 days and that of myelin basic protein at 28 days. ESCS significantly upregulated these markers and increased the percentage of Sox2/CNPase/DAPI-positive cells (newly differentiated oligodendrocytes) at 7 days. Recombinant human bone morphogenetic protein 4 (rhBMP4) markedly downregulated these factors after ESCS. Furthermore, ESCS significantly decreased BMP4 and p-Smad1/5/9 expression after SCI, and rhBMP4 reduced this effect of ESCS. These findings indicate that ESCS enhances the survival and differentiation of oligodendrocytes, protects myelin, and promotes motor functional recovery by inhibiting the BMP4-Smad1/5/9 signaling pathway after SCI.








Similar content being viewed by others
References
Park HW, Oh S, Lee KH, Lee BH, Chang MS. Olig2-expressing mesenchymal stem cells enhance functional recovery after contusive spinal cord injury. Int J Stem Cells 2018, 11: 177–186.
Tran AP, Silver J. Neuroscience. Systemically treating spinal cord injury. Science 2015, 348: 285–286.
Reck TA, Landmann G. Successful spinal cord stimulation for neuropathic below-level spinal cord injury pain following complete paraplegia: a case report. Spinal Cord Ser Cases 2017, 3: 17049.
Huang Q, Duan W, Sivanesan E, Liu S, Yang F, Chen Z, et al. Spinal cord stimulation for pain treatment after spinal cord injury. Neurosci Bull 2019, 35: 527–539.
Darrow D, Balser DY, Netoff T, Krassioukov AV, Phillips AA, Parr AM, et al. Epidural Spinal Cord Stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma 2019, 36: 2325–2336.
Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563: 65–71.
Li H, Dong X, Jin M, Cheng W. The protective effect of spinal cord stimulation postconditioning against spinal cord ischemia/reperfusion injury in rabbits. Neuromodulation 2018, 21: 448–456.
Carhart MR, He J, Herman R, D’Luzansky S, Willis WT. Epidural spinal-cord stimulation facilitates recovery of functional walking following incomplete spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng 2004, 12: 32–42.
Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett 2005, 383: 339–344.
Hachmann JT, Calvert JS, Grahn PJ, Drubach DI, Lee KH, Lavrov IA. Review of epidural spinal cord stimulation for augmenting cough after spinal cord injury. Front Hum Neurosci 2017, 11: 144.
James ND, McMahon SB, Field-Fote EC, Bradbury EJ. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol 2018, 17: 905–917.
Hassannejad Z, Yousefifard M, Azizi Y, Zadegan SA, Sajadi K, Sharif-Alhoseini M, et al. Axonal degeneration and demyelination following traumatic spinal cord injury: A systematic review and meta-analysis. J Chem Neuroanat 2019, 97: 9–22.
Smith AC, Knikou M. A Review on locomotor training after spinal cord injury: reorganization of spinal neuronal circuits and recovery of motor function. Neural Plast 2016, 2016: 1216258.
Zhang C, Zhang G, Rong W, Wang A, Wu C, Huo X. Early applied electric field stimulation attenuates secondary apoptotic responses and exerts neuroprotective effects in acute spinal cord injury of rats. Neuroscience 2015, 291: 260–271.
Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 2001, 81: 871–927.
Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics 2011, 8: 262–273.
Imai T, Katoh H, Suyama K, Kuroiwa M, Yanagisawa S, Watanabe M. Amiloride promotes oligodendrocyte survival and remyelination after spinal cord injury in rats. J Clin Med 2018, 7.
Plemel JR, Keough MB, Duncan GJ, Sparling JS, Yong VW, Stys PK, et al. Remyelination after spinal cord injury: is it a target for repair? Prog Neurobiol 2014, 117: 54–72.
Hesp ZC, Goldstein EZ, Miranda CJ, Kaspar BK, McTigue DM. Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J Neurosci 2015, 35: 1274–1290.
McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci 2001, 21: 3392–3400.
Wang HF, Liu XK, Li R, Zhang P, Chu Z, Wang CL, et al. Effect of glial cells on remyelination after spinal cord injury. Neural Regen Res 2017, 12: 1724–1732.
Li Q, Brus-Ramer M, Martin JH, McDonald JW. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci Lett 2010, 479: 128–133.
Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci 2004, 24: 2182–2190.
Salgado-Ceballos H, Guizar-Sahagun G, Feria-Velasco A, Grijalva I, Espitia L, Ibarra A, et al. Spontaneous long-term remyelination after traumatic spinal cord injury in rats. Brain Res 1998, 782: 126–135.
Papastefanaki F, Matsas R. From demyelination to remyelination: the road toward therapies for spinal cord injury. Glia 2015, 63: 1101–1125.
Alizadeh A, Karimi-Abdolrezaee S. Microenvironmental regulation of oligodendrocyte replacement and remyelination in spinal cord injury. J Physiol 2016, 594: 3539–3552.
Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol 2006, 498: 525–538.
Assinck P, Duncan GJ, Plemel JR, Lee MJ, Stratton JA, Manesh SB, et al. Myelinogenic plasticity of oligodendrocyte precursor cells following spinal cord contusion injury. J Neurosci 2017, 37: 8635–8654.
Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7: 470–482.
Yang XH, Ding Y, Li W, Zhang RY, Wu JL, Ling EA, et al. Effects of electroacupuncture and the retinoid X receptor (RXR) signalling pathway on oligodendrocyte differentiation in the demyelinated spinal cord of rats. Acupunct Med 2017, 35: 122–132.
Shi Y, Shao Q, Li Z, Gonzalez GA, Lu F, Wang D, et al. Myt1L promotes differentiation of oligodendrocyte precursor cells and is necessary for remyelination after lysolecithin-induced demyelination. Neurosci Bull 2018, 34: 247–260.
Fontaine D. Neurosurgical treatment of chronic pain. Rev Prat 2013, 63: 805–809.
Zhu Y, Wu Y, Zhang R. Electro-acupuncture promotes the proliferation of neural stem cells and the survival of neurons by downregulating miR-449a in rat with spinal cord injury. EXCLI J 2017, 16: 363–374.
Geng X, Sun T, Li JH, Zhao N, Wang Y, Yu HL. Electroacupuncture in the repair of spinal cord injury: inhibiting the Notch signaling pathway and promoting neural stem cell proliferation. Neural Regen Res 2015, 10: 394–403.
Wu H, Hu M, Yuan D, Wang Y, Wang J, Li T, et al. Electroacupuncture promotes the proliferation of endogenous neural stem cells and oligodendrocytes in the injured spinal cord of adult rats. Neural Regen Res 2012, 7: 1138–1144.
Becker D, Gary DS, Rosenzweig ES, Grill WM, McDonald JW. Functional electrical stimulation helps replenish progenitor cells in the injured spinal cord of adult rats. Exp Neurol 2010, 222: 211–218.
Hall AK, Miller RH. Emerging roles for bone morphogenetic proteins in central nervous system glial biology. J Neurosci Res 2004, 76: 1–8.
Cheng X, Wang Y, He Q, Qiu M, Whittemore SR, Cao Q. Bone morphogenetic protein signaling and olig1/2 interact to regulate the differentiation and maturation of adult oligodendrocyte precursor cells. Stem Cells 2007, 25: 3204–3214.
Pang EK, Im SU, Kim CS, Choi SH, Chai JK, Kim CK, et al. Effect of recombinant human bone morphogenetic protein-4 dose on bone formation in a rat calvarial defect model. J Periodontol 2004, 75: 1364–1370.
Maeda Y, Ikeuchi M, Wacnik P, Sluka KA. Increased c-fos immunoreactivity in the spinal cord and brain following spinal cord stimulation is frequency-dependent. Brain Res 2009, 1259: 40–50.
Shao Z, Lv G, Wen P, Cao Y, Yu D, Lu Y, et al. Silencing of PHLPP1 promotes neuronal apoptosis and inhibits functional recovery after spinal cord injury in mice. Life Sci 2018, 209: 291–299.
Li ZW, Tang RH, Zhang JP, Tang ZP, Qu WS, Zhu WH, et al. Inhibiting epidermal growth factor receptor attenuates reactive astrogliosis and improves functional outcome after spinal cord injury in rats. Neurochem Int 2011, 58: 812–819.
Hu R, Zhou J, Luo C, Lin J, Wang X, Li X, et al. Glial scar and neuroregeneration: histological, functional, and magnetic resonance imaging analysis in chronic spinal cord injury. J Neurosurg Spine 2010, 13: 169–180.
Li G, Cao Y, Shen F, Wang Y, Bai L, Guo W, et al. Mdivi-1 inhibits astrocyte activation and astroglial scar formation and enhances axonal regeneration after spinal cord injury in rats. Front Cell Neurosci 2016, 10: 241.
Smits H, Ultenius C, Deumens R, Koopmans GC, Honig WM, van Kleef M, et al. Effect of spinal cord stimulation in an animal model of neuropathic pain relates to degree of tactile “allodynia”. Neuroscience 2006, 143: 541–546.
Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional-SCS vs. burst-SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation 2018, 21: 19–30.
Xu Q, Hu D, Duan B, He J. A fully implantable stimulator with wireless power and data transmission for experimental investigation of epidural spinal cord stimulation. IEEE Trans Neural Syst Rehabil Eng 2015, 23: 683–692.
Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995, 12: 1–21.
Li G, Jia Z, Cao Y, Wang Y, Li H, Zhang Z, et al. Mitochondrial division inhibitor 1 ameliorates mitochondrial injury, apoptosis, and motor dysfunction after acute spinal cord injury in rats. Neurochem Res 2015, 40: 1379–1392.
Hong JY, Lee SH, Lee SC, Kim JW, Kim KP, Kim SM, et al. Therapeutic potential of induced neural stem cells for spinal cord injury. J Biol Chem 2014, 289: 32512–32525.
Bernardo A, De Simone R, De Nuccio C, Visentin S, Minghetti L. The nuclear receptor peroxisome proliferator-activated receptor-gamma promotes oligodendrocyte differentiation through mechanisms involving mitochondria and oscillatory Ca2+ waves. Biol Chem 2013, 394: 1607–1614.
Wang LC, Almazan G. Role of sonic hedgehog signaling in oligodendrocyte differentiation. Neurochem Res 2016, 41: 3289–3299.
Shin DC, Ha KY, Kim YH, Kim JW, Cho YK, Kim SI. Induction of endogenous neural stem cells by extracorporeal shock waves after spinal cord injury. Spine (Phila Pa 1976) 2018, 43: E200–E207.
Ossola B, Zhao C, Compston A, Pluchino S, Franklin RJ, Spillantini MG. Neuronal expression of pathological tau accelerates oligodendrocyte progenitor cell differentiation. Glia 2016, 64: 457–471.
Ahn SM, Kim YR, Kim HN, Shin YI, Shin HK, Choi BT. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Sci Rep 2016, 6: 28646.
Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci 2007, 27: 3416–3428.
Lu HZ, Wang YX, Zou J, Li Y, Fu SL, Jin JQ, et al. Differentiation of neural precursor cell-derived oligodendrocyte progenitor cells following transplantation into normal and injured spinal cords. Differentiation 2010, 80: 228–240.
Wislet-Gendebien S, Bruyere F, Hans G, Leprince P, Moonen G, Rogister B. Nestin-positive mesenchymal stem cells favour the astroglial lineage in neural progenitors and stem cells by releasing active BMP4. BMC Neurosci 2004, 5: 33.
Wu B, Sun L, Li P, Tian M, Luo Y, Ren X. Transplantation of oligodendrocyte precursor cells improves myelination and promotes functional recovery after spinal cord injury. Injury 2012, 43: 794–801.
Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J 2004, 4: 16–26.
Lytle JM, Wrathall JR. Glial cell loss, proliferation and replacement in the contused murine spinal cord. Eur J Neurosci 2007, 25: 1711–1724.
Beaumont E, Guevara E, Dubeau S, Lesage F, Nagai M, Popovic M. Functional electrical stimulation post-spinal cord injury improves locomotion and increases afferent input into the central nervous system in rats. J Spinal Cord Med 2014, 37: 93–100.
Huang S, Tang C, Sun S, Cao W, Qi W, Xu J, et al. Protective effect of electroacupuncture on neural myelin sheaths is mediated via promotion of oligodendrocyte proliferation and inhibition of oligodendrocyte death after compressed spinal cord injury. Mol Neurobiol 2015, 52: 1870–1881.
Braun R, Klein R, Walter HL, Ohren M, Freudenmacher L, Getachew K, et al. Transcranial direct current stimulation accelerates recovery of function, induces neurogenesis and recruits oligodendrocyte precursors in a rat model of stroke. Exp Neurol 2016, 279: 127–136.
Kim YR, Kim HN, Ahn SM, Choi YH, Shin HK, Choi BT. Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia. PLoS One 2014, 9: e90000.
Yang Z, Yu H, Rao X, Liu Y, Pi M. Effects of electroacupuncture at the conception vessel on proliferation and differentiation of nerve stem cells in the inferior zone of the lateral ventricle in cerebral ischemia rats. J Tradit Chin Med 2008, 28: 58–63.
Falnikar A, Li K, Lepore AC. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res 2015, 1619: 91–103.
Dusart I, Marty S, Peschanski M. Demyelination, and remyelination by Schwann cells and oligodendrocytes after kainate-induced neuronal depletion in the central nervous system. Neuroscience 1992, 51: 137–148.
Patel JR, Klein RS. Mediators of oligodendrocyte differentiation during remyelination. FEBS Lett 2011, 585: 3730–3737.
Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, et al. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia 2012, 60: 281–294.
Jing JH, Qian J, Zhu N, Chou WB, Huang XJ. Improved differentiation of oligodendrocyte precursor cells and neurological function after spinal cord injury in rats by oscillating field stimulation. Neuroscience 2015, 303: 346–351.
Sozmen EG, Rosenzweig S, Llorente IL, DiTullio DJ, Machnicki M, Vinters HV, et al. Nogo receptor blockade overcomes remyelination failure after white matter stroke and stimulates functional recovery in aged mice. Proc Natl Acad Sci U S A 2016, 113: E8453–E8462.
Uemura MT, Ihara M, Maki T, Nakagomi T, Kaji S, Uemura K, et al. Pericyte-derived bone morphogenetic protein 4 underlies white matter damage after chronic hypoperfusion. Brain Pathol 2018, 28: 521–535.
Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci 2011, 31: 6053–6058.
Hampton DW, Asher RA, Kondo T, Steeves JD, Ramer MS, Fawcett JW. A potential role for bone morphogenetic protein signalling in glial cell fate determination following adult central nervous system injury in vivo. Eur J Neurosci 2007, 26: 3024–3035.
Ramos-Cejudo J, Gutierrez-Fernandez M, Otero-Ortega L, Rodriguez-Frutos B, Fuentes B, Vallejo-Cremades MT, et al. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 2015, 46: 221–228.
Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia 2012, 60: 476–486.
Acknowledgements
This research was supported by the Natural Science Foundation of Liaoning Province (201602277), and the Science and Technology Planning Project of Liaoning Province (LJQ2014091). We thank Barry Patel, PhD, for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest, financial or otherwise.
Rights and permissions
About this article
Cite this article
Li, G., Fan, ZK., Gu, GF. et al. Epidural Spinal Cord Stimulation Promotes Motor Functional Recovery by Enhancing Oligodendrocyte Survival and Differentiation and by Protecting Myelin after Spinal Cord Injury in Rats. Neurosci. Bull. 36, 372–384 (2020). https://doi.org/10.1007/s12264-019-00442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00442-0