Abstract
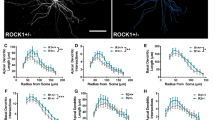
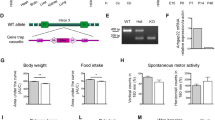
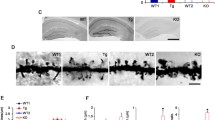
Rho-associated kinases (ROCKs) are serine-threonine protein kinases that act downstream of small Rho GTPases to regulate the dynamics of the actin cytoskeleton. Two ROCK isoforms (ROCK1 and ROCK2) are expressed in the mammalian central nervous system. Although ROCK activity has been implicated in synapse formation, whether the distinct ROCK isoforms have different roles in synapse formation and function in vivo is not clear. Here, we used a genetic approach to address this long-standing question. Both Rock1+/− and Rock2+/− mice had impaired glutamatergic transmission, reduced spine density, and fewer excitatory synapses in hippocampal CA1 pyramidal neurons. In addition, both Rock1+/− and Rock2+/− mice showed deficits in long-term potentiation at hippocampal CA1 synapses and were impaired in spatial learning and memory based on the water maze and contextual fear conditioning tests. However, the spine morphology of CA1 pyramidal neurons was altered only in Rock2+/− but not Rock1+/− mice. In this study we compared the roles of ROCK1 and ROCK2 in synapse formation and function in vivo for the first time. Our results provide a better understanding of the functions of distinct ROCK isoforms in synapse formation and function.







Similar content being viewed by others
References:
Bellot A, Guivernau B, Tajes M, Bosch-Morató M, Valls-Comamala V, Muñoz FJ. The structure and function of actin cytoskeleton in mature glutamatergic dendritic spines. Brain Res 2014, 1573: 1–16.
Bacon C, Endris V and Rappold GA. The cellular function of srGAP3 and its role in neuronal morphogenesis. Mech Dev 2013, 130: 391–395.
Tomasoni R, Repetto D, Morini R, Elia C, Gardoni F, Di Luca M, et al. SNAP-25 regulates spine formation through postsynaptic binding to p140Cap. Nat Commun 2013, 4: 2136.
Amano M, Tsumura Y, Taki K, Harada H, Mori K, Nishioka T, et al. A proteomic approach for comprehensively screening substrates of protein kinases such as Rho-kinase. PLoS One 2010, 5: e8704.
Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol 2003, 15: 590–597.
Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature 2002, 420: 629–635.
Hall A. Rho GTPases and the actin cytoskeleton. Science 1998, 279: 509–514.
Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996, 15: 2208–2216.
Swanger SA, Mattheyses AL, Gentry EG, Herskowitz JH. ROCK1 and ROCK2 inhibition alters dendritic spine morphology in hippocampal neurons. Cell Logist 2015, 5: e1133266.
Zhou Z, Meng Y, Asrar S, Todorovski Z, Jia Z. A critical role of Rho-kinase ROCK2 in the regulation of spine and synaptic function. Neuropharmacology 2009, 56: 81–89.
Schmandke A, Schmandke A, Strittmatter SM. ROCK and Rho: Biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 2007, 13: 454–469.
Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010, 67: 545–554.
Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996, 271: 20246–20249.
Kimura K, Eguchi S. Angiotensin II type-1 receptor regulates RhoA and Rho-kinase/ROCK activation via multiple mechanisms. Focus on “Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells”. Am J Physiol Cell Physiol 2009, 297: C1059–C1061.
Sumi T, Matsumoto K and Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem 2001, 276: 670–676.
Shi J, Wu X, Surma M, Vemula S, Zhang L, Yang Y, et al. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis 2013, 4: e483.
Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 2003, 23: 5043–5055.
Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, et al. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 2005, 168: 941–953.
Zhang Z, Ottens AK, Larner SF, Kobeissy FH, Williams ML, Hayes RL, et al. Direct Rho-associated kinase inhibition [correction of inhibiton] induces cofilin dephosphorylation and neurite outgrowth in PC-12 cells. Cell Mol Biol Lett 2006, 11: 12–29.
Yao L, Chandra S, Toque HA, Bhatta A, Rojas M, Caldwell RB, et al. Prevention of diabetes-induced arginase activation and vascular dysfunction by Rho kinase (ROCK) knockout. Cardiovasc Res 2013, 97: 509–519.
Yin DM, Chen YJ, Lu YS, Bean JC, Sathyamurthy A, Shen C, et al. Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron 2013, 78: 644–657.
Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 1998, 79: 1-4.
Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci 1994, 108: 810–817.
Chan CB, Chen Y, Liu X, Tang X, Lee CW, Mei L, et al. PIKE-mediated PI3-kinase activity is required for AMPA receptor surface expression. EMBO J 2011, 30: 4274–4286.
Kamal A, Ramakers GM, Altinbilek B, Kas MJ. Social isolation stress reduces hippocampal long-term potentiation: Effect of animal strain and involvement of glucocorticoid receptors. Neuroscience 2014, 256: 262–270.
Chen Y, Behnisch T. The role of gamma-secretase in hippocampal synaptic transmission and activity-dependent synaptic plasticity. Neurosci Lett 2013, 554: 16–21.
Bobo-Jiménez V, Delgado-Esteban M, Angibaud J, Sánchez-Morán I, de la Fuente A, Yajeya J, et al. APC/C(Cdh1)-Rock2 pathway controls dendritic integrity and memory. Proc Natl Acad Sci U S A 2017, 114: 4513–4518.
García-Rojo G, Fresno C, Vilches N, Díaz-Véliz G, Mora S, Aguayo F, et al. The ROCK inhibitor fasudil prevents chronic restraint stress-induced depressive-like behaviors and dendritic spine loss in rat hippocampus. Int J Neuropsychopharmacol 2017, 20: 336–345.
Newell-Litwa KA, Badoual M, Asmussen H, Patel H, Whitmore L, Horwitz AR. ROCK1 and 2 differentially regulate actomyosin organization to drive cell and synaptic polarity. J Cell Biol 2015, 210: 225–242.
Uppal N, Puri R, Yuk F, Janssen WG, Bozdagi-Gunal O, Harony-Nicolas H, et al. Ultrastructural analyses in the hippocampus CA1 field in Shank3-deficient mice. Mol Autism 2015, 6: 41.
Segal M. Dendritic spines, synaptic plasticity and neuronal survival: Activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 2010, 31: 2178–2184.
Yin DM, Chen YJ, Liu S, Jiao H, Shen C, Sathyamurthy A, et al. Calcyon stimulates neuregulin 1 maturation and signaling. Mol Psychiatry 2015, 20: 1251–1260.
Zito K. The flip side of synapse elimination. Neuron 2003, 37: 1–2.
Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci U S A 2006, 103: 17961–17966.
Yin DM, Sun XD, Bean JC, Lin TW, Sathyamurthy A, Xiong WC, et al. Regulation of spine formation by ErbB4 in PV-positive interneurons. J Neurosci 2013, 33: 19295–19303.
Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J Neurosci 1989, 9: 2982–2997.
Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol 2009, 186: 85–97.
Huang L, Li Q, Wen R, Yu Z, Li N, Ma L, et al. Rho-kinase inhibitor prevents acute injury against transient focal cerebral ischemia by enhancing the expression and function of GABA receptors in rats. Eur J Pharmacol 2017, 797: 134–142.
Liley AW. The quantal components of the mammalian end-plate potential. J Physiol 1956, 133: 571–587.
Del CJ, Katz B. Potential and resistance changes at the motor endplate. J Physiol 1954, 123: 70P–71P.
Habets RL, Borst JG. Dynamics of the readily releasable pool during post-tetanic potentiation in the rat calyx of Held synapse. J Physiol 2007, 581: 467–478.
Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 1997, 18: 483–491.
Kang S, Ling QL, Liu WT, Lu B, Liu Y, He L, et al. Down-regulation of dorsal striatal RhoA activity and impairment of working memory in middle-aged rats. Neurobiol Learn Mem 2013, 103: 3–10.
Rozic G, Lupowitz Z, Piontkewitz Y, Zisapel N. Dynamic changes in neurexins’ alternative splicing: Role of Rho-associated protein kinases and relevance to memory formation. PLoS One 2011, 6: e18579.
Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron 2011, 70: 863–885.
Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 2012, 17: 142–153.
Meziane H, Khelfaoui M, Morello N, Hiba B, Calcagno E, Reibel-Foisset S, et al. Fasudil treatment in adult reverses behavioural changes and brain ventricular enlargement in Oligophrenin-1 mouse model of intellectual disability. Hum Mol Genet 2016, 25: 2314–2323.
Acknowledgements
We thank Dr. R William Caldwell (Department of Pharmacology and Toxicology, Medical College of Georgia, Augusta, GA, 30912-2300, USA) for providing the transgenic Rock1+/− and Rock2+/− mice. This work was supported by the National Science Foundation of China (81774406 and 31571041), the Guangdong Innovative and Entrepreneurial Research Team Program (Natural Science) (2017KCXTD006), Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2016), and the Scientific Research and Innovation Team Program of Guangzhou University of Chinese Medicine (2017KYTD03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, J., Pan, Y., Zheng, X. et al. Comparative Study of ROCK1 and ROCK2 in Hippocampal Spine Formation and Synaptic Function. Neurosci. Bull. 35, 649–660 (2019). https://doi.org/10.1007/s12264-019-00351-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-019-00351-2