Abstract
Objective
Despite accumulating evidence on a role of immune cells and their associated chemicals in mechanisms of pain, few studies have addressed the potential role of chemokines in the descending facilitation of persistent pain. The present study was undertaken to test the hypothesis that the chemokine (C-C motif) ligand 2 (CCL2) (commonly known as monocyte chemoattractant protein-1) signaling in the rostral ventromedial medulla (RVM), a pivotal structure in brainstem pain modulatory circuitry, is involved in descending pain facilitation in rats.
Methods
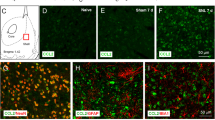
An L5 spinal nerve ligation (SNL) was produced in rats under pentobarbital anesthesia. Western blot and immunohistochemistry were used to detect the expression levels of CCL2 and CCL2 receptor (CCR2), and examine their distributions compared with the neuronal marker NeuN as well as glial markers glial fibrillary acidic protein (GFAP, astroglial) and CD11b (microglial), respectively.
Results
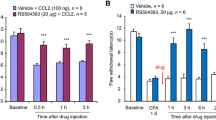
SNL induced an increase in CCL2 expression in the RVM, and this returned to the control level at 4 weeks after injury. The induced CCL2 colocalized with NeuN, but not with GFAP and CD11b. CCR2 was also upregulated by SNL in the RVM, and this increase lasted for at least 4 weeks. CCR2 was colocalized with CD11b but not GFAP. Few RVM neurons also exhibited CCR2 staining. Neutralizing CCL2 with an anti-CCL2 antibody (0.2–20 ng) or injecting RS-102895 (0.1–10 pmol), a CCR2b chemokine receptor antagonist, into the RVM on day 1 after SNL, significantly attenuated the established thermal and mechanical hypersensitivity. In addition, injection of recombinant rat CCL2 (0.03–3 pmol) into the RVM induced dose-dependent hyperalgesia, which was prevented by pretreatment with RS-102895 (10 pmol). Interleukin-1β (IL-1β), a potent inducer of neuronal CCL2, was also selectively upregulated in RVM reactive astrocytes. Injection of IL-1β (120 fmol) into the RVM induced behavioral hyperalgesia, which was blocked by RS-102895 (10 pmol). However, an IL-1 receptor antagonist (3 pmol) did not prevent CCL2 (3 pmol)-induced hyperalgesia. These results suggest that the effect of CCL2 is downstream to IL-1β signaling.
Conclusion
The IL-1β and CCL2-CCR2 signaling cascades play a role in neuron-glia-cytokine interactions and the descending facilitation of neuropathic pain.
Similar content being viewed by others
References
Bonica JJ. Pain research and therapy: Past and current status and future needs. In: Ng LKY, Bonica JJ (Eds.). Pain, Discomfort and Humanitarian Care. Volume 4. New York: Elsevier/North-Holland, 1980: 1–47.
Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci 2002, 25: 319–325.
Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain 2002, 100: 1–6.
Wiertelak EP, Roemer B, Maier SF, Watkins LR. Comparison of the effects of nucleus tractus solitarius and ventral medial medulla lesions on illness-induced and subcutaneous formalin-induced hyperalgesias. Brain Res 1997, 748: 143–150.
Ren K, Dubner R. Descending control mechanisms. In: Bushnell MC, Basbaum AI (Eds.). The Senses: A Comprehensive Reference. Volume 5: Pain. San Diego: Academic Press, 2008: 723–762.
Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glialneuronal interactions contribute to descending pain facilitation. J Neurosci 2008, 28: 10482–10495.
Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med 2005, 257: 139–155.
Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 2007, 10: 1361–1368.
Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther 2010, 126: 56–68.
Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol 2008, 21: 570–579.
Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med 2010, 16: 1267–1276.
White FA, Miller RJ. Insights into the regulation of chemokine receptors by molecular signaling pathways: functional roles in neuropathic pain. Brain Behav Immun 2010, 24: 859–865.
Old EA, Malcangio M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol 2011, 12: 1–7.
Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem 2006, 97: 772–783.
Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, et al. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain 2007, 3: 38.
Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem 2008, 104: 254–263.
Van Steenwinckel J, Reaux-Le Goazigo A, Pommier B, Mauborgne A, Dansereau MA, Kitabgi P, et al. CCL2 released from neuronal synaptic vesicles in the spinal cord is a major mediator of local inflammation and pain after peripheral nerve injury. J Neurosci 2011, 31: 5865–5875.
White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, et al. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A 2005, 102: 14092–14097.
Tsakiri N, Kimber I, Rothwell NJ, Pinteaux E. Differential effects of interleukin-1 alpha and beta on interleukin-6 and chemokine synthesis in neurones. Mol Cell Neurosci 2008, 38: 259–265.
Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A 2003, 100: 7947–7952.
Zhang J, Shi XQ, Echeverry S, Mogil JS, De Koninck Y, Rivest S. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J Neurosci 2007, 27: 12396–12406.
Knerlich-Lukoschus F, Juraschek M, Blömer U, Lucius R, Mehdorn HM, Held-Feindt J. Force-dependent development of neuropathic central pain and time-related CCL2/CCR2 expression after graded spinal cord contusion injuries of the rat. J Neurotrauma 2008, 25: 427–448.
Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci 2009, 1: 4096–4108.
Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol 2006, 96: 2189–2199.
Belkouch M, Dansereau MA, Réaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, et al. The Chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a GβΓ-dependent mechanism. J Neurosci 2011, 31: 18381–18390.
Serrano A, Paré M, McIntosh F, Elmes SJ, Martino G, Jomphe C, et al. Blocking spinal CCR2 with AZ889 reversed hyperalgesia in a model of neuropathic pain. Mol Pain 2010, 6: 90.
Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience 2009, 159: 780–786.
Thacker MA, Clark AK, Bishop T, Grist J, Yip PK, Moon LD, et al. CCL2 is a key mediator of microglia activation in neuropathic pain states. Eur J Pain 2009, 13: 263–272.
Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, et al. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience 2007, 149: 706–714.
Padi SS, Shi XQ, Zhao YQ, Ruff MR, Baichoo N, Pert CB, et al. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain 2011. [Epub ahead of print]
Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, et al. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci U S A 2002, 99: 10276–10281.
Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50: 355–363.
Paxinos P, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. New York: Academic Press, 2008.
Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32: 77–88.
Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Beh 1999, 67: 711–716.
Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, et al. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci 2004, 24: 9161–9173.
Graves DT, Jiang Y. Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med 1995, 6: 109–118.
Tran PB, Miller RJ. Chemokine receptors: signposts to brain devel opment and disease. Nat Rev Neurosci 2003, 4: 444–455.
Thibeault I, Laflamme N, Rivest S. Regulation of the gene encoding the monocyte chemoattractant protein 1 (MCP-1) in the mouse and rat brain in response to circulating LPS and proinflammatory cytokines. J Comp Neurol 2001, 434: 461–477.
Banisadr G, Gosselin RD, Mechighel P, Kitabgi P, Rostène W, Parsadaniantz SM. Highly regionalized neuronal expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) in rat brain: evidence for its colocalization with neurotransmitters and neuropeptides. J Comp Neurol 2005, 489: 275–292.
Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-α-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia 2010, 58: 1871–1880.
Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res 2004, 48: 463–469.
van der Meer P, Ulrich AM, Gonźalez-Scarano F, Lavi E. Immunohistochemical analysis of CCR2, CCR3, CCR5, and CXCR4 in the human brain: potential mechanisms for HIV dementia. Exp Mol Pathol 2000, 69: 192–201.
Banisadr G, Quéraud-Lesaux F, Boutterin MC, Pélaprat D, Zalc B, Rostène W, et al. Distribution, cellular localization and functional role of CCR2 chemokine receptors in adult rat brain. J Neurochem 2002, 81: 257–269.
Gosselin RD, Varela C, Banisadr G, Mechighel P, Rostene W, Kitabgi P, et al. Constitutive expression of CCR2 chemokine receptor and inhibition by MCP-1/CCL2 of GABA-induced currents in spinal cord neurones. J Neurochem 2005, 95: 1023–1034.
Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, et al. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J Neurosci 2009, 29: 8051–8062.
De Leo JA, Tawfik VL, LaCroix-Fralish ML. The tetrapartite synapse: path to CNS sensitization and chronic pain. Pain 2006, 122: 17–21.
Ren K, Wei F. Neuron-glial interaction in descending modulation of persistent pain, In: Schmidt RF, Gebhart GF (Eds.). Encyclopedic Reference of Pain. Volume 3. 2nd ed. Heidelberg New York: Springer-Verlag, 2011.
Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci 2009, 30: 229–241.
El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med 2007, 13: 432–438.
Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci 2007, 27: 6006–6018.
Gu M, Miyoshi K, Dubner R, Guo W, Zou S, Ren K, et al. Spinal 5-HT(3) receptor activation induces behavioral hypersensitivity via a neuronal-glial-neuronal signaling cascade. J Neurosci 2011, 31: 12823–12836.
Baamonde A, Hidalgo A, Menéndez L. Involvement of glutamate NMDA and AMPA receptors, glial cells and IL-1β in the spinal hyperalgesia evoked by the chemokine CCL2 in mice. Neurosci Lett 2011, 502: 178–181.
Flügel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, et al. Neuronal MCP-1 expression in response to remote nerve injury. J Cereb Blood Flow Metab 2001, 21: 69–76.
Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, et al. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci 2010, 30: 8624–8636.
Author information
Authors and Affiliations
Corresponding author
Additional information
These authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, W., Wang, H., Zou, S. et al. Chemokine signaling involving chemokine (C-C motif) ligand 2 plays a role in descending pain facilitation. Neurosci. Bull. 28, 193–207 (2012). https://doi.org/10.1007/s12264-012-1218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-012-1218-6