Abstract
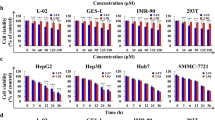
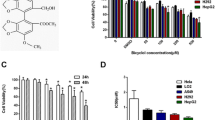
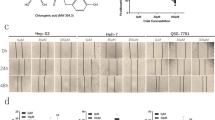
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and one of the leading causes of cancer-related death. Cilostazol, an antiplatelet drug, elicits anticancer effects on human squamous cell carcinoma and colon cancer cells. We previously reported that cilostazol protects normal mature hepatocytes from alcohol-induced apoptotic cell death. In addition, cilostazol stimulates liver regeneration after hepatectomy. Therefore, this study evaluated whether cilostazol elicits pro- or anti-proliferative effects on HCC using Hep3B and SK-Hep1 cells. Cilostazol inhibited proliferation of HCC cells by inducing apoptosis. Additionally, cilostazol induced G0/G1 cell cycle arrest and decreased expression of cyclin D1 and proliferating cell nuclear antigen. Activation of AMP-activated protein kinase (AMPK) and inhibition of extracellular signal-regulated kinase (ERK) and AKT signaling were associated with the anti-proliferative effect of cilostazol. LY294002 and PD98059, inhibitors of AKT and ERK, respectively, enhanced the anti-proliferative effect of cilostazol. By contrast, inhibition of AMPK using compound C or AMPK-targeting siRNA abolished the anti-proliferative effect of cilostazol. Moreover, AMPK inhibition reversed the down-regulation of AKT/EKR induced by cilostazol, indicating negative cross-talk between AMPK and AKT/ERK. These findings provide evidence that cilostazol exerts anti-tumor activity in HCC by counter-regulating AMPK and AKT/ERK signaling. Taken together, our findings suggest that cilostazol may provide clinical benefits in HCC patients by selectively targeting HCC cells without interfering with liver function.
Similar content being viewed by others
References
Tabrizian, P., S. Roayaie, and M. E. Schwartz (2014) Current management of hepatocellular carcinoma. World J. Gastroenterol. 20: 10223–10237.
Tejeda-Maldonado, J., I. García-Juárez, J. Aguirre-Valadez, A. González-Aguirre, M. Vilatobá-Chapa, A. Armengol-Alonso, F. Escobar-Penagos, A. Torre, J. F. Sánchez-Ávila, and D. L. Carrillo-Pérez (2015) Diagnosis and treatment of hepatocellular carcinoma: An update. World J. Hepatol. 7: 362–376.
Gomez, D., H. Z. Malik, G. K. Bonney, V. Wong, G. J. Toogood, J. P. A. Lodge, and K. R. Prasad (2007) Steatosis predicts postoperative morbidity following hepatic resection for colorectal metastasis. Br. J. Surg. 94: 1395–1402.
Nordlinger, B., H. Sorbye, B. Glimelius, G J. Poston, P. M. Schlag, P. Rougier, W. O. Bechstein, J. N. Primrose, E. T. Walpole, M. Finch-Jones, D. Jaeck, D. Mirza, R. W. Parks, L. Collette, M. Praet, U. Bethe, E. Van Cutsem, W. Scheithauer, and T. Gruenberger (2008) Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 371: 1007–1016.
Shi, J. H. and P. D. Line (2014) Effect of liver regeneration on malignant hepatic tumors. World J. Gastroenterol. 20: 16167–16177.
Poon, R. T., S. T. Fan, C. B. O’Suilleabhain, and J. Wong (2002) Aggressive management of patients with extrahepatic and intrahepatic recurrences of hepatocellular carcinoma by combined resection and locoregional therapy. J. Am. Coll. Surg. 195: 311–318.
He, C., T. Wu, and Y. Hao (2018) Anlotinib induces hepatocellular carcinoma apoptosis and inhibits proliferation via Erk and Akt pathway. Biochem. Biophys. Res. Commun. 503: 3093–3099.
Wang, J., Z. Luo, T. Yao, W. Li, and J. Pu (2019) LINC00707 promotes hepatocellular carcinoma progression through activating ERK/JNK/AKT pathway signaling pathway. J. Cell. Physiol. 234: 6908–6916.
Matter, M. S., T. Decaens, J. B. Andersen, and S. S. Thorgeirsson (2014) Targeting the mTOR pathway in hepatocellular carcinoma: current state and future trends. J. Hepatol. 60: 855–865.
Grabinski, N., F. Ewald, B. T. Hofmann, K. Staufer, U. Schumacher, B. Nashan, and M. Jücker (2012) Combined targeting of AKT and mTOR synergistically inhibits proliferation of hepatocellular carcinoma cells. Mol. Cancer. 11: 85.
Ito, Y., Y. Sasaki, M. Horimoto, S. Wada, Y. Tanaka, A. Kasahara, T. Ueki, T. Hirano, H. Yamamoto, J. Fujimoto, E. Okamoto, N. Hayashi, and M. Hori (1998) Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 27: 951–958.
Hoffmann, K., L. Shibo, Z. Xiao, T. Longerich, M. W. Büchler, and P. Schemmer (2011) Correlation of gene expression of ATP-binding cassette protein and tyrosine kinase signaling pathway in patients with hepatocellular carcinoma. Anticancer Res. 31: 3883–3890.
Liu, L., Y. Cao, C. Chen, X. Zhang, A. McNabola, D. Wilkie, S. Wilhelm, M. Lynch, and C. Carter (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 66: 11851–11858.
Wang, S. S., Y. H. Chen, N. Chen, L. J. Wang, D. X. Chen, H. L. Weng, S. Dooley, and H. G. Ding (2017) Hydrogen sulfide promotes autophagy of hepatocellular carcinoma cells through the PI3K/Akt/mTOR signaling pathway. Cell Death Dis. 8: e2688.
Yang, S. and G. Liu (2017) Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol. Lett. 13: 1041–1047.
Akula, S. M., S. L. Abrams, L. S. Steelman, M. R. Emma, G. Augello, A. Cusimano, A. Azzolina, G. Montalto, M. Cervello, and J. A. McCubrey (2019) RAS/RAF/MEK/ERK, PI3K/PTEN/AKT/mTORC1 and TP53 pathways and regulatory miRs as therapeutic targets in hepatocellular carcinoma. Expert Opin. Ther. Targets. 23: 915–929.
Mihaylova, M. M. and R. J. Shaw (2011) The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13: 1016–1023.
Ruderman, N. B., D. Carling, M. Prentki, and J. M. Cacicedo (2013) AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Invest. 123: 2764–2772.
Cordero, M. D., M. R. Williams, and B. Ryffel (2018) AMP-activated protein kinase regulation of the NLRP3 inflammasome during aging. Trends Endocrinol. Metab. 29: 8–17.
Umezawa, S., T. Higurashi, and A. Nakajima (2017) AMPK: Therapeutic target for diabetes and cancer prevention. Curr. Pharm. Des. 23: 3629–3644.
Cao, W., J. Li, Q. Hao, J. V. Vadgama, and Y. Wu (2019) AMP-activated protein kinase: a potential therapeutic target for triple-negative breast cancer. Breast Cancer Res. 21: 29.
Merlen, G., G. Gentric, S. Celton-Morizur, M. Foretz, J. E. Guidotti, V. Fauveau, J. Leclerc, B. Viollet, and C. Desdouets (2014) AMPKα1 controls hepatocyte proliferation independently of energy balance by regulating Cyclin A2 expression. J. Hepatol. 60: 152–159.
Huang, J., D. Zhang, L. Lin, R. Jiang, J. Dai, L. Tang, Y. Yang, P. Ge, B. Wang, and L. Zhang (2018) Potential roles of AMP-activated protein kinase in liver regeneration in mice with acute liver injury. Mol. Med. Rep. 17: 5390–5395.
Jiang, X., H. Y. Tan, S. Teng, Y. T. Chan, D. Wang, and N. Wang (2019) The role of AMP-activated protein kinase as a potential target of treatment of hepatocellular carcinoma. Cancers. 11: 647.
Kawaguchi, T., M. Hayakawa, H. Koga, and T. Torimura (2015) Effects of fucoidan on proliferation, AMP-activated protein kinase, and downstream metabolism- and cell cycle-associated molecules in poorly differentiated human hepatoma HLF cells. Int. J. Oncol. 46: 2216–2222.
Ferretti, A. C., F. Hidalgo, F. M. Tonucci, E. Almada, A. Pariani, M. C. Larocca, and C. Favre (2019) Metformin and glucose starvation decrease the migratory ability of hepatocellular carcinoma cells: targeting AMPK activation to control migration. Sci. Rep. 9: 2815.
Cheng, J., T. Huang, Y. Li, Y. Guo, Y. Zhu, Q. Wang, X. Tan, W. Chen, Y. Zhang, W. Cheng, T. Yamamoto, X. Jing, and J. Huang (2014) AMP-activated protein kinase suppresses the in vitro and in vivo proliferation of hepatocellular carcinoma. PLoS One. 9: e93256.
Kwon, H. Y., J. H. Kim, B. Kim, S. K. Srivastava, and S. H. Kim (2018) Regulation of SIRT1/AMPK axis is critically involved in gallotannin-induced senescence and impaired autophagy leading to cell death in hepatocellular carcinoma cells. Arch. Toxicol. 92: 241–257.
Park, S. Y., Y. K. Lee, H. J. Kim, O. J. Park, and Y. M. Kim (2016) AMPK interacts with β-catenin in the regulation of hepatocellular carcinoma cell proliferation and survival with selenium treatment. Oncol. Rep. 35: 1566–1572.
Chiang, P. C., S. C. Lin, S. L. Pan, C. H. Kuo, I. L. Tsai, M. T. Kuo, W. C. Wen, P. Chen, and J. H. Guh (2010) Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: a crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 79: 162–171.
Ishii, H., T. Aoyama, H. Takahashi, Y. Kumada, D. Kamoi, T. Sakakibara, N. Umemoto, S. Suzuki, A. Tanaka, Y. Ito, and T. Murohara (2016) Treatment with cilostazol improves clinical outcome after endovascular therapy in hemodialysis patients with peripheral artery disease. J. Cardiol. 67: 199–204.
Ratchford, E. V. (2017) Medical management of claudication. J. Vasc. Surg. 66: 275–280.
Fujii, T., H. Obara, K. Matsubara, N. Fujimura, H. Yagi, T. Hibi, Y. Abe, M. Kitago, M. Shinoda, O. Itano, M. Tanabe, Y. Masugi, M. Sakamoto, and Y. Kitagawa (2017) Oral administration of cilostazol improves survival rate after rat liver ischemia/reperfusion injury. J. Surg. Res. 213: 207–214.
Jeon, B. H., Y. H. Lee, M. R. Yun, S. H. Kim, B. W. Lee, E. S. Kang, H. C. Lee, and B. S. Cha (2015) Increased expression of ATP-binding cassette transporter A1 (ABCA1) as a possible mechanism for the protective effect of cilostazol against hepatic steatosis. Metabolism. 64: 1444–1453.
Kabil, S. L. (2018) Beneficial effects of cilostazol on liver injury induced by common bile duct ligation in rats: Role of SIRT1 signaling pathway. Clin. Exp. Pharmacol. Physiol. 45: 1341–1350.
Xie, X., X. Xu, C. Sun, and Z. Yu (2018) Protective effects of cilostazol on ethanol-induced damage in primary cultured hepatocytes. Cell Stress Chaperones. 23: 203–211.
Lee, Y. J., M. S. Shu, J. Y. Kim, Y. H. Kim, K. H. Sim, W. J. Sung, and J. R. Eun (2019) Cilostazol protects hepatocytes against alcohol-induced apoptosis via activation of AMPK pathway. PLoS One. 14: e0211415.
von Heesen, M., S. Dold, S. Müller, C. Scheuer, O. Kollmar, M. K. Schilling, M. D. Menger, and M. R. Moussavian (2015) Cilostazol improves hepatic blood perfusion, microcirculation, and liver regeneration after major hepatectomy in rats. Liver Transpl. 21: 792–800.
Strowitzki, M. J., S. Dold, M. von Heesen, C. Körbel, C. Scheuer, M. R. Moussavian, M. K. Schilling, O. Kollmar, and M. D. Menger (2014) The phosphodiesterase 3 inhibitor cilostazol does not stimulate growth of colorectal liver metastases after major hepatectomy. Clin. Exp. Metastasis. 31: 795–803.
Uzawa, K., A. Kasamatsu, T. Baba, K. Usukura, Y. Saito, K. Sakuma, M. Iyoda, Y. Sakamoto, K. Ogawara, M. Shiiba, and H. Tanzawa (2013) Targeting phosphodiesterase 3B enhances cisplatin sensitivity in human cancer cells. Cancer Med. 2: 40–49.
Kangawa, Y., T. Yoshida, K. Maruyama, M. Okamoto, T. Kihara, M. Nakamura, M. Ochiai, Y. Hippo, S. M. Hayashi, and M. Shibutani (2017) Cilostazol and enzymatically modified isoquercitrin attenuate experimental colitis and colon cancer in mice by inhibiting cell proliferation and inflammation. Food Chem. Toxicol. 100: 103–114.
Jiao, P., Y. S. Zhou, J. X. Yang, Y. L. Zhao, Q. Q. Liu, C. Yuan, and F. Z. Wang (2013) MK-2206 induces cell cycle arrest and apoptosis in HepG2 cells and sensitizes TRAIL-mediated cell death. Mol. Cell. Biochem. 382: 217–224.
Lee, C. H., Y. J. Hung, Y. S. Shieh, C. Y. Chien, Y. J. Hsu, C. Y. Lin, C. F. Chiang, C. L. Huang, and C. H. Hsieh (2017) Cilostazol inhibits uremic toxin-induced vascular smooth muscle cell dysfunction: role of Axl signaling. Am. J. Physiol. Renal. Physiol. 312: F398–F406.
Yoo, A. R., S. H. Koh, G. W. Cho, and S. H. Kim (2010) Inhibitory effects of cilostazol on proliferation of vascular smooth muscle cells (VSMCs) through suppression of the ERK1/2 pathway. J. Atheroscler. Thromb. 17: 1009–1018.
Zheng, L., W. Yang, F. Wu, C. Wang, L. Yu, L. Tang, B. Qiu, Y. Li, L. Guo, M. Wu, G. Feng, D. Zou, and H. Wang (2013) Prognostic significance of AMPK activation and therapeutic effects of metformin in hepatocellular carcinoma. Clin. Cancer Res. 19: 5372–5380.
Chan, K. M., C. F. Kuo, J. T. Hsu, M. J. Chiou, Y. C. Wang, T. H. Wu, C. F. Lee, T. J. Wu, H. S. Chou, and W. C. Lee (2017) Metformin confers risk reduction for developing hepatocellular carcinoma recurrence after liver resection. Liver Int. 37: 434–441.
Seo, Y. S., Y. J. Kim, M. S. Kim, K. S. Suh, S. B. Kim, C. J. Han, Y. J. Kim, W. I. Jang, S. H. Kang, H. J. Tchoe, C. M. Park, A. J. Jo, H. J. Kim, J. A. Choi, H. J. Choi, M. N. Polak, and M. J. Ko (2016) Association of metformin use with cancer-specific mortality in hepatocellular carcinoma after curative resection: A nationwide population-based study. Medicine. 95: e3527.
Schulte, L., B. Scheiner, T. Voigtländer, S. Koch, N. Schweitzer, S. Marhenke, P. Ivanyi, M. P. Manns, T. Rodt, J. B. Hinrichs, A. Weinmann, M. Pinter, A. Vogel, and M. M. Kirstein (2019) Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int. 39: 714–726.
Acknowledgement
This work was supported by research grants from Daegu Catholic University in 2018 (No.20185003).
The authors have no potential conflicts of interest to disclose. Neither ethical approval nor informed consent was required for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sim, K.H., Shu, MS., Kim, S. et al. Cilostazol Induces Apoptosis and Inhibits Proliferation of Hepatocellular Carcinoma Cells by Activating AMPK. Biotechnol Bioproc E 26, 776–785 (2021). https://doi.org/10.1007/s12257-021-0002-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-021-0002-8