Summary
Although no game-changing studies in bladder cancer were presented this year at ASCO, we saw a series of interesting data including follow-up updates, exploratory analyses and promising results of phase I/II trials. An up-date analysis of the JAVELIN 100 Bladder study indicated a benefit of avelumab maintenance treatment irrespective of the response to platin-based first-line treatment and administration of second-line treatment. A new HER2-directed antibody drug conjugate, disitamab vedotin, showed promising results with high-response rates in HER2 high and low bladder cancer. Longer follow-up data confirmed the significant benefit of enfortumab-vedotin in the third-line treatment setting.
Similar content being viewed by others
Update JAVELIN 100 Bladder study
At ASCO 2022, the longest follow-up data (median 38 months) of the JAVELIN 100 Bladder study were presented. In this randomized prospective phase III study, patients received a platin-based regimen for 4–6 cycles, and upon achieving complete/partial remission, or disease stabilization, were randomized (n = 700) to either avelumab 800 mg maintenance treatment or best supportive care (BSC). With extended follow-up (median, ≥ 38 months in both arms for all patients; data cutoff, June 4, 2021), overall survival as the primary endpoint remained significantly longer in the avelumab plus BSC versus BSC alone arm in all randomized patients. The avelumab maintenance approach significantly prolonged overall survival (primary endpoint: 23.8 months versus 15 months, hazard ratio 0.76) and progression-free survival (secondary endpoint: 5.5 months versus 2.1 months, hazard ratio 0.54), respectively. Moreover, in the PD-L1 positive subgroup, a median overall survival of 30.9 months versus 18.5 months was reported (hazard ratio 0.69, p < 0.001). Exploratory analysis indicated that the avelumab maintenance therapy was superior irrespective of response during the platin-based first-line therapy. In addition, exploratory analysis demonstrated that the benefit holds true irrespective of receiving a second-line treatment upon progression (abstracts #4559, #4560). In summary, avelumab maintenance therapy is clearly considered as the current standard of care in patients responding or stabilizing upon platinum-based first-line treatment.
Novel HER2-directed approaches in bladder cancer
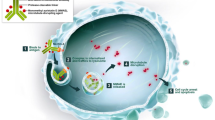
A series of smaller single arm phase I/II studies introduced a new antibody-drug conjugate (ADC) called disitamab vedotin (RC-48). The mechanism of action of this novel HER2-directed ADC includes a highly specific antibody with high binding affinity to HER2 receptor, a cysteine-maleimide linker, and MMAE (Monomethyl-Auristatin E) as the cytotoxic payload. In their presentation (abstract #4518), Sheng et al. presented data showing a response rate of 62.2% in the group of classical HER2 IHC3+ and HER2 IHC2+ FISH-positive patients (n = 45), and 39.3% in the group of HER2 IHC2+ FISH-negative patients (n = 53). Median progression-free survival (PFS) was 5.9 months, median overall survival (OS) was 14.2 months, respectively. Remarkably, 64.5% of these patients received at least two previous treatment lines. Xu et al. presented data including 19 patients with HER2 negative or low (IHC1+) disease. Where there was no response reported for the HER2-negative group, an encouraging response rate of 38.5% in the IHC1+ group (n = 13) was reported. These findings are important, as it is estimated that about two thirds of bladder cancer patients will have IHC1+ (HER2 low) disease. Finally, the first combinatorial data with the PD‑1 inhibitor toripalimab showed an impressive response rate of 71.8% in a predominantly first-line treatment population (~ 60% of patients). Summarizing these early phase I/II data, disitamab vedotin (RC-48) seems to be a promising approach in HER2-positive disease and ongoing phase III trials will evaluate this targeted approach in bladder cancer.
Long-term outcome in EV-301 enfortumab-vedotin study
As a reminder, the EV-301 study was a randomized phase III trial comparing enfortumab-vedotin to monochemotherapy (vinflunine or a taxane) in the third-line setting after failure of a platin-based combination therapy and one immune checkpoint inhibitor. This year at ASCO 2022, the 24-month median follow-up data were presented, confirming the significant improvement of overall survival (hazard ratio 0.70, 12.9 months versus 8.9 months, p < 0.001) and progression-free survival (hazard ratio 0.63, 5.5 months versus 3.7 months, p < 0.001) in favor of enfortumab-vedotin. The objective response rate improved from 18.6% to 41.3% (p < 0.001), thus confirming enfortumab-vedotin as the standard of care in the third-line setting. Rash, neuropathy and hyperglycemia were confirmed as the most frequent > grade 2 adverse events. Of note, the ongoing EV-302 phase III trial will clarify the role of combining enfortumab-vedotin with pembrolizumab compared to standard platin-based first-line treatment. In summary, enfortumab-vedotin is recommended as the current third-line standard of care in patients upon platinum-based first-line and immune checkpoint inhibitor second-line/maintenance treatment.
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. Pichler received honoraria for advisory boards and scientific presentations from Astellas, Merck, Pfizer, MSD, BMS, Roche, Janssen.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pichler, M. Best of ASCO 2022—bladder cancer. memo 15, 273–274 (2022). https://doi.org/10.1007/s12254-022-00847-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12254-022-00847-0