Abstract
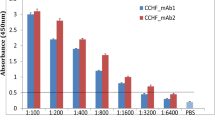
A monoclonal antibody (MAb) specific for the bluetongue virus (BTV) group specific antigen (VP7) was characterized for its reactivity with purified virus and recombinant BTV VP7 (rVP7) protein and its suitability for use in the sandwich ELISA. The MAb, designated as 5B5 was specific to VP7 and belongs to IgG2a subclass and was selected for the development of the sELISA in this study. The MAb had a titer of 1:25 with BTV and 1:2 with the rVP7 protein. The sELISA is based on capturing of BTV antigen with VP7 specific MAb followed by detection using BTV polyclonal antiserum raised in rabbits. The assay was evaluated with six cell culture adapted serotypes of BTV that have been isolated from India, 1, 2, 15, 17, 18 and 23. The assay could detect BTV antigen as early as day 8 in blood. It was also successfully applied for the detection of BTV group specific antigen in clinical samples of blood, washed RBCs, buffy coat and plasma. A total of 102 field samples from animals, suspected of being infected with BTV, were tested and 29.42% were positive. The blood samples were also amplified in cell culture which improved the sensitivity of the assay. Results confirmed that the sELISA is rapid and specific.
Similar content being viewed by others
References
Anderson J. 1984. Use of monoclonal antibodies in a blocking ELISA to detect group specific antibodies to bluetongue virus. J Immunol Methods, 7(1): 139–149.
Bhanuprakash V, Hosamani M, Singh N K,et al. 2007. Comparative efficacy of indirect ELISA with competitive ELISA in the detection of bluetongue virus. Indian J Anim Sci, 77(7): 53–56.
Clavijo A, Heckert R A, Dulac G C,et al. 2000. Isolation and identification of bluetongue virus. J Virol Methods, 87(1–2): 13–23.
Fauquet C M, Mayo M A, Maniloff J,et al. 2005. In: Virus Taxonomy. Eighth Report of the International Committee on Taxonomy of Viruses. Amsterdam: Elsevier-Academic Press, p515–520.
Hawkes R A, Kirkland P D, Sanders D A,et al. 2000. Laboratory and field studies of an antigen capture ELISA for bluetongue virus. J Virol Methods, 85(1–2): 137–149.
Hosseini M, Hawkes R A, Kirkland P D,et al. 1998. Rapid screening of embryonated chicken eggs for bluetongue virus infection with an antigen capture enzyme linked immunosorbent assay. J Virol Methods, 75: 39–46.
Huismans H, van dar walt N T, Cloete M,et al. 1987: Isolation of capsid protein of bluetongue virus that induces a protective immune response in sheep. Virology, 157(1): 172–179.
Laemmli U K. 1970. Cleavage of structural protein during the analysis of head of bacteriophageT4. Nature, 227: 680.
Lelli R, Portanti O, Langella V,et al. 2003. Production of a competitive ELISA kit for the serological diagnosis of bluetongue disease. Vet Ital, 39(47): 5–13.
Mahrt C R, Osburn B I. 1986. Experimental bluetongue virus infection of sheep, effect of vaccination: pathologic, immunofluorescent, and ultra-structural studies. Am J Vet Res, 47:1198–1203.
Mecham J O. 1993. Detection of bluetongue virus from blood of infected sheep by use of an antigen-capture enzyme-linked immunosorbent assay after amplification of the virus in cell culture. Am J Vet Res, 54(3): 370–372.
Mertens P P C, Burroughs J N, Anderson J. 1987. Purification and properties of virus particles, infectious sub-viral particles, and cores of bluetongue virus serotypes 1 and 4. Virology, 157: 375–386.
Nunamaker R A, Ellis J A, Wingington J G,et al. 1992. The detection of intracellular bluetongue virus particles within ovine erythrocytes. Comp Biochem Physiol, 101(3): 471–476.
Orru G, Ferrando M L, Meloni M,et al. 2006. Rapid detection and quantitation of bluetongue virus (BTV) using a molecular beacon fluorescent probe assay. J Virol Methods, 137(1): 34–42.
Pathak K B, Biswas S K, Tembhurne P A,et al. 2008. Prokaryotic expression of truncated VP7 of bluetongue virus (BTV) and reactivity of the purified recombinant protein with all BTV type-specific sera. J Virol Methods, 152(1–2): 6–12.
Peters J H, Baumgarten H. 1992. In: Monoclonal antibodies (Springer Laboratory ed.), Springer Laboratory, USA.
Portanti O, Luciani M, Ronchi G F. 2005. Rapid detection of bluetongue virus in blood and organ samples using a capture enzyme-linked Immunosorbent assay. Vet Ital, 41(1): 15–21.
Reed L J, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg, 27: 493–497.
Roy P. 1989. Bluetongue virus genetics and genome structure. Virus Res, 13: 179–206.
Sambrook J, Fritsch E F, Maniatis T. 1989. In: Molecular cloning. A Laboratory Manual, 2 ed., Cold Spring Harbor Laboratory Press, USA.
Singh R P, Bandyopadhyay S K, Sreenivasa B P,et al. 2004. Production and characterization of monoclonal antibodies to peste des petits ruminants (PPR) virus. Vet Res Commun, 28: 623–639.
Sreenivasulu D, Subba Rao M V, Reddy Y N,et al. 2004. Overview of bluetongue disease, viruses, vectors, surveillance and unique features: the Indian sub-continent and adjacent regions. Vet Ital, 40(3): 73–77.
Stanislawek W, Lunt R, Blacksell S,et al. 1996. Detection by ELISA of bluetongue antigen directly in the blood of experimentally infected sheep. Vet Microbiol, 52: 1–12.
Wuijckhuise V L, Dercksen D, Muskens J,et al. 2006. Bluetongue in The Netherlands; Description of the first clinical cases and differential diagnosis. Common symptoms just a little different and in too many herds. Tijdschr Diergeneeskd, 131(18): 649–654.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gandhale, P.N., Bhanuprakash, V., Balamurugan, V. et al. Detection of bluetongue virus group-specific antigen using monoclonal antibody based sandwich ELISA. Virol. Sin. 25, 390–400 (2010). https://doi.org/10.1007/s12250-010-3160-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-010-3160-y