Abstract
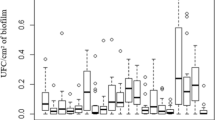
The intestinal microbiota has enormous impact on the health and performance of horses. Staphylococci belong in the phylum Firmicutes, and their occurrence, especially of methicillin-resistant strains and species, has been reported in horses previously. Moreover, biofilm formation is one of the virulence factors; it has been not completely studied in fecal coagulase-negative staphylococci (CoNS) from horses. Therefore, this study was focused on biofilm formation by various species of fecal CoNS from horses because it has been never reported before. In addition, their antibiotic profile was tested. Horses (42) of various breeds from Slovakia/Poland were sampled. Variability in the species of CoNS was detected in feces of horses. Thirty-two strains were identified by using the MALDI-TOF system and classified into nine species and three subspecies of CoNS: Staphylococcus capitis, S. cohnii subsp. cohnii, S. cohnii subsp. urealyticus, S. cohnii subsp. casei, S. epidermidis, S. haemolyticus, S. pasteuri, S. sciuri, S. vitulinus, S. warneri, and S. xylosus. The most frequent species was S. vitulinus. Twenty-two strains showed high biofilm production; 10 strains showed low-grade biofilm production. The highest biofilm formation was measured in the species S. xylosus. Eleven strains (of 32) were methicillin-resistant; the others were susceptible to methicillin.
Similar content being viewed by others
References
Anderson AC, Jonas D, Huber I, Karygianni L, Wolber J, Hellwig E, Arwelier N, Vach K, Wittmer A, Ahmed Ali A (2016) Enterococcus faecalis from food, clinical specimenes and oral sites: prevalence of virulence factors in associatyion with biofilm formation. Front Microbiol 6:1534
Bailey SR, Reinemeyer CR, Morgan SJ, Brooks AC, Longhofer SL, Elliot J (2009) Plasma concentrations of endotoxin and platelet activation in the developmental stage of oligofructose-induced laminitis. Vet Immunol Immunolpathol 129:167–173. https://doi.org/10.1016/j.vetmn.2008.11.009
Busscher JF, van Duijkeren E, Sloet van Oldruitenborh-Oosterbaan M (2006) The prevalence of methicillin-resistant staphylococci in healthy horses in the Netherlands. Vet Microbiol 113:131–136
Chaieb K, Chehab O, Zmantar T, Rouabhia M, Mahdouani K, Bakhrouf A (2007) In vitro effect of pH and ethanol on biofilm formation by clinical ica-positive Staphylococcus epidermidis strains. Ann Microbiol 57:431–437. https://doi.org/10.1007/BF03175085
Clinical laboratory standard institute guideline, CLSI (2016) Performance standards for antimicrobial susceptibility testing M100S, 26th edn
Costa MC, Weese JS (2012) The equine intestinal microbiome. Animal health research reviews/conference of research workers in animal Diseases, pp 1–8
Costa MC, Arroyo LG, Allen-Vercoe E, Stämpli TR, Kim PT, Sturgeon A, Weese Scott J (2012) Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS One 7:e41484
Davies D (2003) Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov 2:114–122
De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer KH, Whitman WB (1999) Bergeys manual of systematic bacteriology. In: The Firmicutes, vol 3, 2nd edn. Springer
Dougal K, de la Fuente, Harris PA, Girdwood SE, Pinloche E, Newbold CJ (2013) Identification of a core bacterial community within the large intestine of the horse. PLoS One 8:e77660. https://doi.org/10.1371/journal.pone.0077660
Drogoul C, de Fombelle A, Julliand V (2001) Feeding and microbial disorders in horses:2:effect of three hay; grain ratios on digesta passage rate and digestibility in ponies. J Equine Vet Sci 21:487–491. https://doi.org/10.1016/S0737-0806(01)70211-0
Eucast (European Committee on Antimicrobial Susceptibility Testing) (2016) Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0 2016. http://www.eucast.org
Lauková A (1994) Antimicrobial susceptibility of ruminal coagulase-negative staphylococci. Microbiologica 17:123–132
Lauková A, Kandričáková A (2015) Staphylococci detected in faecal samples of common pheasants. Int J Curr Microbiol Appl Sci 4:788–797
Lauková A, Simonová M, Strompfová V, Štyriak I, Ouwehand AC, Várady M (2008) Potential of enterococci isolated from horses. Anaerobe 14:234–236. https://doi.org/10.1016/j.anaerobe.2008-04-002
Lauková A, Strompfová V, Pogány Simonová M, Szabóová R (2011) Methicillin-resistant Staphylococcus xylosus isolated from horses and their sensitivity to enterocins and herbal substances. Slovak J Anim Sci 44:167–171
Lauková A, Kandričáková A, Buňková L, Pleva P, Ščerbová J (2017) Effect of lantibiotic gallidermin against biogenic amines producing faecal staphylococci from ostriches and pheasants. Folia Microbiol 62:229–235
Martins A, De Cunha ML (2007) Methicillin resistance in Staphylococcus aureus and coagulase-negative staphylococci: epidemiological and molecular aspects. Microbiol Immunol 51:787–795
Moodley A, Guardabassi L (2009) Clonal spread of methicillin-resistant coagulase-negative staphylococci among horses, personnel and environmental sites at equine facilities. Vet Microbiol 137:397–401
Pyorälä S, Taponen S (2009) Coagulase-negative staphylococci-emerging mastitis pathogens. Vet Microbiol 134:3–8
Schleifer KH, Bell JA (2015) Staphylococcus. In: Bergey DH, Harrison FC, Breed RS, Hammer BW, Huntoon FM (eds) Bergeys manual of systematic archaea and bacteria 1. https://doi.org/10.1002/9781118960608.gbm00569
Schoster A, Weese JS, Guardabassi L (2014) Probiotic use in horses-what is the evidence for their clinical efficacy? J Vet Intern Med 28:1640–1652
Singhal N, Kumar M, Kanaujia PK, Vird JS (2015) Maldi-Tof mass spectrometry: an emerging technology for microbial identification and diagnosis. Front Microbiol 6:791. https://doi.org/10.3389/fmicb.2015.00791
Slížová M, Nemcová R, Maďar M, Hádryová J, Gancarčíková S, Popper M, Pistl J (2015) Analysis of biofilm formation by intestinal lactobacilli. Can J Microbiol 61:437–466. https://doi.org/10.1139/cjm-2015-0007
Soares R, Colombo AP (2014) Correlation between biofilm formation and gelE, esp and agg genes in Enterococcus sp. clinical isolates. Virulence 5:634–637
Takashi T, Satoh I, Kikuchi N (1999) Phylogenetic relationship of 38 taxa of the genus Staphylococcus based on 16S rRNA gene sequence analysis. Int J Syst Bacteriol 49:725–728
Wu MT, Burnham CAD, Westblade LF, Dien bard J, lawhon SD, Wallace MA, Stanley T, Burd E, Hindler J, Humphries RM (2016) Evaluation of oxacillin and cefoxitin disk and MIC breakpoints for prediction of methicillin resistance in human and veterinary isolates of Staphylococcus intermedius group. J Clin Microbiol 54:535541
Yasuda R, Kawano J, Matsuo E, Masuda T, Shimizu A, Anzai T, Hashikura S (2002) Distribution of mecA-harboring staphylococci in healthy mares. J Vet Med Sci 64:821–827
Acknowledgments
We are grateful to Mrs. Margita Bodnárová for her skillful laboratory work. We also thank Dr. Eva Styková from the University of Veterinary Medicine and Pharmacy in Košice (UVMP, Slovakia) for providing us with fecal samples from Slovak horses, and Dr. Radomíra Nemcová also from UVMP for assisting with the microtiter plate assay. Finally we are thankful to Mr. Andrew Billingham for his English language control.
Funding
These results were achieved by using financial support from the Slovak Scientific Agency VEGA, the projects Vega 2/0012/16 and 2/0006/17. Part of the project was also supported by the Statutory Research Fund of the Kielanowski Institute of Animal Physiology and Nutrition, Polish Academy of Sciences, Jablonna, Poland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bino, E., Lauková, A., Ščerbová, J. et al. Fecal coagulase-negative staphylococci from horses, their species variability, and biofilm formation. Folia Microbiol 64, 719–726 (2019). https://doi.org/10.1007/s12223-019-00684-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-019-00684-5