Abstract
Nosocomial infections are a major cause of morbidity and mortality among neonates admitted to neonatal intensive care units (NICUs). The aim of this paper was to describe an outbreak of Escherichia coli among infants admitted to the NICU of the General Hospital “Dr. Manuel Gea Gonzalez” in May of 2008. The isolated E. coli strains were identified using standard biochemical methods. The susceptibilities of these strains were analysed by determining their minimal inhibitory concentrations. Following this, their molecular relationships to each other were assessed by pulsed field gel electrophoresis (PFGE) analysis and corroborated by serology. Twelve E. coli strains were isolated from blood, urine, or indwelling catheter samples from five cases of preterm infants within a 3-day period. Patients were admitted to the NICU of the general hospital and, during the outbreak, developed sepsis caused by E. coli. For four of the patients, the average age was 23 days, while one patient was a 3-month-old infant. Prior to sepsis, the infants had received assisted ventilation and hyperalimentation through a central venous catheter. Two profiles were observed by PFGE; profile A was identified as the outbreak’s cause and an outcome of cross-infection, while profile B showed genetic differences but serologically it was identified as part of the same serotype. We conclude that E. coli colonised the patients through horizontal transmission. A focal source of the microorganism in this outbreak was not identified, but cross-transmission through handling was the most probable route.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nosocomial infections are a major cause of morbidity and mortality among neonates admitted to neonatal intensive care units (NICUs). Nosocomial infections are estimated to cause 1.6 million deaths annually, 40 % of the global burden of child mortality, and are the major cause of neonatal deaths in developing countries (WHO 1993). Premature birth is the most common reason for admission to NICUs. The critically ill neonate is particularly prone to life-threatening bacterial infections because prematurity carries risk factors such as low gestational age, low birth body mass, the presence of underlying diseases, and lengthy hospitalisations; these factors result in a need for intensive care (parenteral nutrition, oxygen therapy, mechanical ventilation, and catheterisation). All of these factors contribute to higher rates of infection than in paediatric and adult intensive care units. These bacterial infections can be acquired by exposure to microbes that colonise the maternal genital tract (vertical transmission) or by exposure to unhygienic care practices and environments. Late-onset sepsis, or sepsis that occurs after 3 days of age, is an important problem in very low birth body mass (VLBBM) infants (Stoll et al. 2002a).
Infections with Gram-negative bacilli are a major cause of neonatal mortality. Escherichia coli is the second most important Gram-negative bacilli that causes bloodstream infections (Stoll et al. 2002a; Larson et al. 2005). There have been several attempts to characterise the epidemiology of nosocomial infections by identifying risk factors for their acquisition. The environmental source of an outbreak is difficult to determine (Casolari et al. 2005). However, it has been clearly identified with certainty in milk, breast pumps, extracted breast milk, bottles of parenteral food additives, and intravenous and topical solutions. This work aimed to assess the genetic relationship between the E. coli strains involved in the outbreak by means of molecular typing with pulsed field gel electrophoresis (PFGE). This allowed for the assessment of the clonal relationship of the outbreak isolates, tracing horizontal transmission between newborns, assessment of the degree of cross-transmission of these strains, and the identification of somatic antigens using serology.
Patients and methods
Background
The General Hospital “Dr. Manuel Gea Gonzalez” in Mexico is a 206-bed second level care centre. The NICU has 20 beds for the admission of critically ill newborn infants. Approximately 660 newborns are admitted to the NICU each year.
Bacterial strains
The 12 strains of E. coli used in this study were isolated from five NICU patients in the hospital. Three of the patients were VLBBM infants (401–1,500 g) who developed respiratory distress syndrome (RDS) and late-onset sepsis. These patients had a positive result for one or more blood cultures of E. coli obtained after 72 h of life. Samples were obtained either from the blood, urine or an indwelling catheter from each patient. Milk formulas (NAN-1 and pre-NAN, Nestlé, México City, México) were also analysed. An incidence case was defined as an infant infected or colonised with E. coli. Each case included in this work was first identified by surveillance cultures. The isolated strains were preserved in 30 % glycerol at −70 °C until use.
Identification and antimicrobial susceptibility test
Samples from the NICU were processed by the clinical microbiology laboratory. The identification and antimicrobial susceptibilities for each sample were determined by the MicroScan4 automated lecture system (Dade Behring, West Sacramento, CA, USA) using standardised minimum concentration breakpoints.
Pulsed-field gel electrophoresis
Horizontal transmission of E. coli was assessed by examining the endonuclease restriction digestion patterns of all isolates by PFGE. Bacteria were embedded in agarose according to the method described in PulseNet (www.pulsenetinternational.org), then lysed in situ with lysozyme and proteinase K (20 mg/mL). Next, the chromosomal DNA was digested by the restriction endonucleases, XbaI and BlnI (Boehringer, Mannheim), and resolved by PFGE performed with the CHEF-Mapper apparatus (Bio-Rad, Hercules, CA, USA). The universal size standard, Salmonella Braederup DNA restricted with XbaI, was used as molecular size marker (Hunter et al. 2005). Each profile was visually inspected and compared with the other profiles after resolution on a 1 % agarose gel (Seakem Gold agarose) and staining with ethidium bromide. The gels were run at 12 °C, 6 V/cm and with a 120° switch angle for 19 h, with a pulse time that ramped from 2.16 to 54.17 s. The analysis of band patterns was performed using the Bioimage band analyser software. Isolates were considered to be the same strain if their restriction fragment band profiles were identical. They were interpreted as closely related if their PFGE patterns showed two to three band differences and unrelated if their PFGE patterns showed seven or more band differences (Tenover et al. 1995).
Serotyping
All strains were agglutinated by dilution in a microplate with rabbit antiserum against the 186 E. coli somatic antigens. The flagella antigens were determined using 56 antisera to H antigens antisera (Orskov and Orskov 1975).
Results
Identification and antimicrobial susceptibility
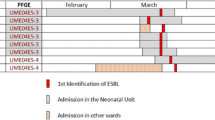
A total of 12 E. coli strains were isolated from five patients during a 3-day period from blood (two isolates), a catheter (one isolate), urine (two isolates) and bronchial secretions (seven isolates). E. coli was not found in the milk formulas NAN-1 and pre-NAN during the same period. The isolates were identified with internal numbers as follows: patient 1, two isolates (I1, I2); patient 2, three isolates (I3, I4, I5); patient 3, four isolates (I6, I7, I8, I9); patient 4, two isolates (I10, I11); and patient 5, one isolate (I12). All isolates included in pattern A were resistant to aminoglycosides, ampicillin/sulbactam, third-generation cephalosporins and fluoroquinolones but susceptible to β-lactam antibiotics and trimethoprim/sulfamethoxazole; pattern B was susceptible to amikacin, carboxypenicillin and β-lactam antibiotics but resistant to piperacilin, ampicillin and trimethoprim/sulfamethoxazole. All isolates showed the same serological profile as O20:H9. The features of the cases are listed chronologically in Table 1.
Determination of horizontal transmission by PFGE
Horizontal transmission was assessed by analysing isolates by PFGE. This analysis revealed that the clinical isolates of E. coli associated with this outbreak displayed two different patterns (Fig. 1a and b). A clonal relationship among the isolates of pattern A was outstanding; this profile was displayed in nine of the isolates (Fig. 1a and b). Therefore, the pattern A strain was regarded as the epidemic strain and the cause of the outbreak. Pattern B corresponded to a nosocomial strain from the urinary tract of a patient who was not infected by the epidemic strain (pattern A).
PFGE patterns of E. coli outbreak isolates. a PFGE patterns of DNA digested with XbaI. 1, 7, and 15 Salmonella Branderup H9812 digested with XbaI (the global standard (PulseNet) molecular size marker); 3, 4, 5, 6, 8, 9, 10, 11, 12, 13, and 14 epidemic clones shared by four patients in NICU identified as pattern A; 2 unique clone from an infant in NICU identified as pattern B. b PFGE patterns of DNA digested with BlnI. 1, 7, and 15 Salmonella Branderup H9812 molecular size marker (digested with XbaI); 3, 4, 5, 6, 8, 9, 10, 11, 12, 13, and 14 clone shared by four patients in NICU identified as pattern A; 2 unique clone isolated from an infant in NICU identified as pattern B
Discussion
Neonates are extremely vulnerable to life-threatening bacterial infections, as they are innately immuno-compromised as result of premature birth. In this study, three out of five neonates who developed septicaemia weighed less than 1,500 g, and their gestational age was less than 33 weeks. They all underwent intensive care, including receiving a central venous catheter. Three of the patients were put on a mechanical ventilator. Traditionally, neonatal infections have been divided into early-onset infections related to maternal risk factors and birth canal acquisition (those presenting within the first 72 h of life) and late-onset infections related to acquisition in the home or hospital environment (those presenting after 72 h of life; Zaidi et al. 2005). Gram-negative bacteria are the most important organisms responsible for early-onset sepsis. The amount of infections caused by these bacteria has increased toward the end of the last decade (Dent and Toltzis 2003).
E. coli is the second most important Gram-negative bacillus with respect to bloodstream infections and hand carriage by healthcare workers, especially with respect to carriage on hands and artificial nails in neonatal intensive care units (Gupta et al. 2004; Larson et al. 2005). The most common reservoir for this pathogen appears to be the gastrointestinal tract of colonised patients, even though it is usually absent in the normal neonatal intestinal flora. Patient-to-patient transmission is facilitated by transient or persistent carriage on the hands of healthcare workers. Infection outbreaks caused by Gram-negative bacilli are increasing and are well recognised because of the morbidity and mortality rates associated with these infections (Stoll et al. 2002b). These facts were obvious during this outbreak, in which four cases began in less than 24 h and no environmental sources could be detected.
In this outbreak, two PFGE profiles were observed. The first, profile A, was identified as the epidemic strain and was resistant to ampicillin and aminoglycosides. Studies had previously shown that E. coli resistant to ampicillin is correlated with intrapartum ampicillin treatment. The E. coli with profile A was only sensitive to third generation cephalosporins (Stoll et al. 2002b). Profile B displayed identical serology; however, it was an unrelated isolate from the urinary tract of patient 5 who had an infection onset at the same time as the onset of profile A infection. This patient was admitted to the NICU at the time of the outbreak. The observation that profile B, isolated from patient 5, had shown identical serology is in agreement with the considerations of Selander et al. (1986). Moreover, Moreno et al. (2005) determined that E. coli strains of urinary tract origin that cause bacteremia are a distinct group to extraintestinal pathogenic E. coli (Moreno et al. 2005). In these cases, the risk factors linked to premature birth together with the intrinsic virulence of the pathogen contributed to the onset of serious invasive infections and the fatal outcomes in two of the cases.
The availability of molecular typing methods is of great potential value in determining the phylogeny of bacteria from a putative outbreak. PFGE remains the method of choice for identifying and comparing different strains amongst the molecular epidemiological techniques used and leads to strain identification and determination of the outbreak source. The most significant drawback of other methods is that they may lead to a false impression of strain heterogeneity in an outbreak. For this reason, PFGE was used to confirm that the outbreak was caused by clonally related isolates and to assess the degree of cross-transmission of these strains.
Profile A was identified in the E. coli isolated from three neonates on the first day of the outbreak and 24 h later in the E. coli isolated from patient 4. Consequently, one of these patients was presumed to be the cross-infection source. Nonetheless, the mode of infection in the source infant could not be established. Pattern B was recovered from patient 5, who was not colonised by profile A. The PFGE patterns indicated that we had identified two genetically distinct E. coli strains.
On the other hand, serotyping identified the specific somatic and flagella determinants and revealed that, despite the genetic differences observed using PFGE, the isolated strains were clones of the same O:H serotype. Epidemiological investigation may have failed to reveal a common source, but the serology results strongly suggested that there was a common source of infection.
We were urged to assess whether milk could have been the E. coli source. Given that E. coli does not survive well on the skin and in environmental reservoirs, it was more likely that the source was within the NICU. Additionally, the environmental sources in the NICU were under strict surveillance and we had observed negative cultures from the reconstituted formula and breast milk. The fact that patients 1, 2, and 3 had been colonised the first day allowed us to posit that transmission of the organism was most likely through the hands of a hospital staff member. We were unable to demonstrate this directly in the current study. We envisioned that milk formula was the possible source, especially due to the account of personnel having been identified as having a role in cross-transmission in a previous Candida parapsilopsis outbreak (Hernández-Delgado et al. 2009; Perlman et al. 2007; Hernández-Castro et al. 2010). Hospital staff as the source of an infection outbreak has also been reported elsewhere involving Salmonella in Tennessee (Boehmer et al. 2009).
The early detection of colonised or infected patients and the prompt implementation of infection control measures were crucial for success in the containment of the nosocomial epidemic. Proper hand hygiene was the most important infection control activity for our health care workers in preventing the transmission of organisms in the neonatal intensive care unit, as mentioned elsewhere (Hernández-Castro et al. 2010). We anticipate that measures towards outbreak prevention will not require much extra effort and that the observations and results described herein may aid the rational development of more infection control strategies to contain the reservoirs of resistant Gram-negative organisms in the NICU.
References
Boehmer TK, Bamberg WM, Ghost TS, Cronquist A, Fornof ME, Cichon MK, Gershman K, Vogt RL (2009) Health care-associated outbreak of Salmonella Tennessse in a neonatal intensive care unit. Am J Infect Control 37:49–55
Casolari C, Pecorari M, Fabio G, Cattani S, Venturelli C, Piccinini L, Tamassia MG, Gennari W, Sabbatini AM, Leporati G, Marchegiano P, Rumpianesi F, Ferrari F (2005) A simultaneous outbreak of Serratia marcescens and Klebsiella pneumoniae in a neonatal intensive care unit. J Hosp Infect 61:312–320
Dent A, Toltzis P (2003) Descriptive and molecular epidemiology of Gram-negative bacilli infections in the neonatal intensive care unit. Curr Opin Infect Dis 16:279–283
Gupta A, Della-Latta P, Todd B, San Gabriel P, Haas J, Wu F, Rubenstein D, Saiman L (2004) Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect Control Hosp Epidemiol 25:210–215
Hernández-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, Moncada-Barrón D, Alvarez-Verona E, Hernández-Delgado L, Torres-Narváez P, Lavalle-Villalobos A (2010) Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr 169:783–787
Hernández-Delgado L, Lavalle-Villalobos A, García-Torres D, Torres-Narváez P, Vázquez-Zavala G, Flores-Nava G (2009) Post intervention reduction of bacteremias related to vascular catheters into neonatal and pediatric intensive care. Bol Med Hosp Infant Mex 66:419–424
Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, Wrigley D, Barrett T, Ribot E (2005) Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol 43:1045–1050
Larson EL, Cimiotti JP, Haas J, Nesin M, Allen A, Della-Latta P, Saiman L (2005) Gram-negative bacilli associated with catheter-associated and non-catheter-associated bloodstream infections and hand carriage by healthcare workers in neonatal intensive care units. Pediatr Crit Care Med 6:457–461
Moreno E, Planells I, Prats G, Planes AM, Moreno G, Andreu A (2005) Comparative study of Escherichia coli virulence determinants in strains causing urinary tract bacteremia versus strains causing pyelonephritis and other sources of bacteremia. Diagn Microbiol Infect Dis 53:93–99
Orskov F, Orskov I (1975) Escherichia coli O:H serotypes isolated from human blood: prevalence of the K1 antigen with technical details of O and H antigenic determination. Acta Pathol Microbiol Scand 83:595–600
Perlman SE, Saiman L, Larson EL (2007) Risk factors for late-onset health care-associated bloodstream infections in patients in neonatal intensive care units. Am J Infect Control 35:177–182
Selander RK, Korhonen TK, Väisänen-Rhen V, Williams PH, Pattison PE, Caugant DA (1986) Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun 52:213–222
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK (2002a) Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network Pediatrics. Pediatrics 110:285–291
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Koroners SB, Shankaran S, Laptook AR, Stevenson DK, Papile L, Poole K (2002b) Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 347:240–247
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239
World Health Organization (1993) Mother–baby package: a road map for implementation in countries. Geneva, WHO. Available from: https://apps.who.int/rht/documents/MSM94-11/9411.htm
Zaidi AKM, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA (2005) Hospital-acquired neonatal infections in developing countries. Lancet 365:1175–1188
Acknowledgments
This work was partially supported by grant CONACyT-87586 and CONACYT-100343 from the Consejo Nacional de Ciencia y Tecnologia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrillo-Casas, E.M., Suástegui-Urquijo, Z., Arroyo-Escalante, S. et al. E. coli outbreak in a neonate intensive care unit in a general hospital in Mexico City. Folia Microbiol 58, 229–234 (2013). https://doi.org/10.1007/s12223-012-0202-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-012-0202-x