Abstract
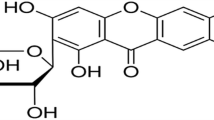
Silk yarn was dyed with morin (2′,3,4′,5,7-pentahydroxyflavone) by using alum as mordant. In order to optimize the process, three methods of dyeing involving: pre-mordanting, simultaneous mordanting, and post-mordanting were assessed and compared with a mordant-free process. The adsorption of alum-morin dye onto silk fibers indicated that the adsorption capacities were significantly affected by pH, the initial dye concentration, and temperature. The initial dye adsorption rates of alum-morin dye on silk before equilibrium was reached increased with higher dyeing temperatures. The pseudo second-order kinetic model was indicated for alum-morin dyeing (simultaneous mordanting) of silk at pH 4.0 with an activation energy (E a ) of 45.26 kJ/mol. The value of the enthalpy of activation (ΔH #) for alum-morin dyeing on silk at pH 4.0 was −31.29 kJ/mol. Also, the free energy (ΔG o) and entropy changes (ΔS o) for alum-morin dyeing on silk were −17.73 kJ/mol and −45.7 J/molK, respectively, consistent with a spontaneous and exothermic adsorption process.
Similar content being viewed by others
References
R. H. M. J. Lemmens and N. Wulijarni-Soetjipto, “Plant Resources of South-East Asia 3: Dye and Tannin-Producing Plants”, Prosea, Bogor Indonesia, 1992.
M. Moeyes, “Natural Dyeing in Thailand”, White Lotus, Bangkok, 1993.
T. Bechtold, A. Turcanu, E. Ganglberger, and S. Geissler, J. Clean. Prod., 11, 499 (2003).
R. Bhuyan and C. N. Saikia, Bioresour. Technol., 96, 363 (2005).
R. Raisanen, P. Nousiainen, and P. H. Hynninen, Text. Res. J., 71, 1016 (2001).
R. Shanker and P. S. Vankar, Dyes Pigm., 74, 464 (2007).
M. Chairat, S. Rattanaphani, J. B. Bremner, and V. Rattanaphani, Dyes Pigm., 64, 231 (2005).
A. C. Gutierrez and M. H. Gehlen, Spectrochim. Acta A., 58, 83 (2002).
C. Septhum, V. Rattanaphani, and S. Rattanaphani, Suranaree J. Sci. Tech., 14, 91 (2007).
C. Septhum, J. Morgan, L. Hick, J. B. Bremner, S. Rattanaphani, and V. Rattanaphani, Anal. Sci., 23, 1209 (2007).
R. M. Christie, R. R. Mather, and R. H. Wardman, “The Chemistry of Colour Application”, Blackwell Science, Oxford, 2000.
F. Capitan, E. Manzano, J. L. Vilchez, and L. F. Capitan-Vallvey, Anal. Sci., 5, 549 (1989).
S. Lagergren, Kungliga Svenska Vetenskapsakademiens. Handlingar, 24(4), 1 (1989).
M. S. Chiou and H. Y. Li, J. Hazard. Mater., B93, 233 (2002).
M. S. Chiou and H. Y. Li, Chemosphere, 50, 1095 (2003).
M. Doğan and M. Alkan, Chemosphere, 50, 517 (2003).
Y. S. Ho and G. McKay, Chem. Eng. J., 70, 115 (1998).
İ Uzun, Dyes Pigm., 70, 76 (2006).
F. C. Wu, R. L. Tseng, and R. S. Juang, Water Res., 35, 613 (2001).
M. Chairat, S. Rattanaphani, J. B. Bremner, and V. Rattanaphani, Dyes Pigm., 76, 435 (2008).
Y. S. Ho and G. McKay, Process Biochem., 34, 451 (1999).
G. Blanchard, M. Maunaye, and G. Martin, Water Res., 18, 1501 (1984).
M. Ozacar and I. A. Sengil, Process Biochem., 40, 565 (2005).
K. J. Laidler, J. H. Meiser, and B. C. Sanctuary, “Physical Chemistry”, Houghton Mifflin, Boston, 2003.
J. E. House, “Principles of Chemical Kinetics”, WC Brown Publishers, Dubuque, IA, 1997.
H. Nollet, M. Roels, P. Lutgen, P. V. Meeren, and W. Verstraete, Chemosphere, 53, 655 (2003).
A. E. Ofomaja, Chem. Eng. J., 26, 35 (2007).
M. Al-Ghouti, M. A. M. Khraisheh, M. N. M. Ahmad, and S. Allen, J. Colloid Interface Sci., 287, 6 (2005).
T. K. Kim, Y. A. So, and Y. J. Lim, Dyes Pigm., 67, 229 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Septhum, C., Rattanaphani, S., Bremner, J.B. et al. An adsorption study of alum-morin dyeing onto silk yarn. Fibers Polym 10, 481–487 (2009). https://doi.org/10.1007/s12221-009-0481-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12221-009-0481-2