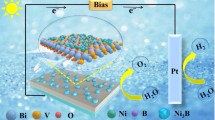
Abstract
Bismuth vanadate is a promising photoanode material for photoelectrochemical (PEC) water splitting, but its activity and stability need to be further improved. In this work, we synthesized Ni-doped BiVO4 abundant with V4+ species and oxygen defects through an in situ electrodeposition method. The effective doping can decrease the particle size of BiVO4 and lead to the formation of V4+ species/oxygen defects. Accordingly, the doped and defective BiVO4 showed high optical absorption and rapid charge transfer, and further showed much higher PEC activity than pure BiVO4. Specifically, 5-Ni-BiVO4 exhibits the highest activity in PEC water splitting, with a photocurrent of 2.39 mA/cm2 at 1.23 V versus RHE (the reversible hydrogen electrode), which is 2.5 times higher than pure BiVO4 (0.94 mA/cm2), and much higher incident photon-to-current efficiency (IPCE) value of 45% (while only 25% for BiVO4 at ca. 400 nm). This work provides an in situ method for the development of a high-performance photoanode.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As the energy crisis and environmental pollution have become serious issues in the past decades [1], sustainable energy needs to be explored, and the utilization of solar energy has drawn much attention recently [2,3,4]. Specifically, photoelectrochemical (PEC) water splitting has been regarded as a promising pathway to convert solar energy to hydrogen fuel [5].
Since Fujishima and Honda [6] first applied TiO2 as a photoanode material for PEC water splitting, much research has been carried out to explore the active photoanodes, such as WO3, Fe2O3, ZnO, BiVO4 [7,8,9,10,11,12]. Among them, bismuth vanadate (m-BiVO4) is an ideal material, attributed to its suitable band gap of 2.4 eV [13,14,15,16] and the ideal PEC current density of 7.6 mA/cm2 under solar light [11].
However, the rapid recombination of the photo-induced electron–hole pairs in BiVO4 restricts its PEC properties. Recently, several methods have been applied to improve its PEC performance, such as morphology control [15, 16], heterojunction [17,18,19,20], surface modification [12, 21], and element doping [22,23,24,25,26,27]. Among them, doping by metal ions is a promising approach to tune the electronic and band structures, which may also cause surface defects and further improve the charge separation [26,27,28,29,30,31]. Recently, Ni ions were applied to narrow the band gap of BiVO4 [32,33,34,35], and the atom radii difference between Ni2+ (0.78 Å) and Bi3+ (1.03 Å) could lead to abundant surface defects, which are beneficial to optical absorption and charge transfer [36].
In this work, we used an in situ electrodeposition method to fabricate a Ni-doped BiVO4 photoanode for PEC water splitting. The doping of Ni ions into the BiVO4 lattice decreases the particle size and causes abundant V4+ species and oxygen defects, which shorten the migration length of charge carriers and enhance optical absorption. Therefore, 5-Ni-BiVO4 exhibits very high PEC performance, with a photocurrent of 2.39 mA/cm2 at 1.23 V versus RHE (the reversible hydrogen electrode) and an incident photon-to-current conversion efficiency (IPCE) value of 45% (at 400 nm).
Experimental Section
Materials
Bi(NO3)3·H2O, Ni(NO3)2·6H2O, VO(acac)2, KI, p-benzoquinone, KOH, and absolute ethanol were all obtained from Aladdin Chemicals Co., Ltd. (China). Dimethyl sulfoxide (DMSO) and HNO3 were purchased from Tianjin Jiangtian Chemical Institute. Milli-Q ultrapure water (> 18 mΩ/cm) was used in all experiments. All reagents were of analytical grade and used without further purification.
Synthesis of Doped BiVO4
0.4 mol/L KI solution (pH = 1.7), Bi(NO3)3·H2O (0.97 g), and Ni(NO3)2·6H2O (0%, 3%, 5%, 10% of Bi source) were dissolved in ultrapure water, which was then mixed with 20 mL of absolute ethanol containing 0.497 g p-benzoquinone (as an electrolyte). Then, a piece of fluorine-doped tin oxide (FTO), a saturated Ag/AgCl electrode, and a platinum counter were used as a working electrode, reference electrode, and counter electrode in a three-electrode cell for electrodeposition, respectively. The electrodeposition of BiOI electrode was then operated potentiostatically at − 0.1 V versus Ag/AgCl for 3 min.
Then, 0.14 mL of DMSO solution containing 0.106 g VO(acac)2 was dripped on the BiOI electrode, which was dried in a vacuum at 40 °C for 8 h. Afterward, the electrodes were calcined at 450 °C for 2 h with an increasing rate of 2 °C/min to obtain BiVO4. Then, 1.0 mol/L KOH was used to rinse the surface V2O5. The Ni-doped BiVO4 with varied proportions was named as 3-Ni-BiVO4, 5-Ni-BiVO4, and 10-Ni-BiVO4.
Characterization
X-ray diffraction (XRD) characterization was applied to analyze the crystalline phase of samples with a diffractometer by Germany Brooke AXSCO. A field emission scanning electron microscope (FE-SEM, Hitachi S-4800) was used to investigate the morphology. High-resolution transmission electron microscopy (HR-TEM, Tecnai G2 F-20) and the attached energy dispersive spectrometer (EDS) were applied to obtain the microstructures and the elemental distribution. The X-ray photoelectron spectrum (XPS) was used to characterize the surface composition of samples, which was conducted with a PHI-1600 X-ray photoelectron spectroscope equipped with Al Kα radiation. Raman spectra were carried out with a Raman spectrometer (DXR Microscope), and the excitation source was a green semiconductor laser (532 nm). Steady-state photoluminescence (PL) spectra were measured by a Horiba Jobin–Yvon Fluorolog 3-21 with the excitation light at 420 nm. The UV–Vis diffuse reflectance spectra (UV–Vis DRS) were recorded on a Shimadzu U-2600 spectrometer equipped with a 60-mm-diameter integrating sphere of BaSO4. The concentration of Ni was detected by inductively coupled plasma-mass spectrometry (ICP-MS) (ICP-MS XSERIES 2, Thermo Fisher Scientific).
PEC Tests
A typical three-electrode cell and a CHI 660E electrochemical workstation were applied to evaluate the PEC performance of the BiVO4-based photoanodes. The simulated solar illumination source was a 300 W Xe arc lamp (100 mW/cm2, PLS-SXE300UV, Beijing Trusttech. Co. Ltd). The electrolyte for the photocurrent measurements was a 0.2 mol/L Na2SO4 aqueous solution. Linear sweep voltammetry (LSV) was monitored while sweeping the potential to the positive direction with a scan rate of 10 mV/s. The potential versus Ag/AgCl reference electrode was converted to the reversible hydrogen electrode (RHE) according to the Nernst equation: E (vs. RHE) = E (vs. Ag/AgCl) + 0.0591 pH + 0.197. Electrochemical impedance spectroscopy (EIS) measurement was taken with a sinusoidal ac perturbation of 10 mV applied over the frequency range of 0.1–105 Hz.
Moreover, the IPCE was then determined and calculated as follows [37]:
where λ is the wavelength (nm), J(λ) is the photocurrent density (mA/cm2), and P(λ) is the incident power density of the monochromatic light (mW/cm2).
Results and Discussion
Crystal Structure and Morphology
As shown in Fig. 1a, pure BiVO4 and Ni-doped BiVO4 samples all showed the typical diffraction peaks of monoclinic scheelite BiVO4 (JCPDS No. 14-0688, space group 15, C2h), with other peaks referring to FTO glass (SnO2, JCPDS No. 41-1445), indicating the absence of nickel oxide species. As shown in the magnified spectra (Fig. 1b), the left shift of the diffraction peak should be owing to the substitution of Bi sites by Ni cations [38], indicating the effective Ni doping in BiVO4.
In the Raman spectra (Fig. 1c), the characteristic vibration peaks of all samples refer to the crystal structure of monoclinic scheelite BiVO4, and the Ni doping gradually decreased the crystallization of BiVO4, which may have caused surface defects. The peak at 215.9 cm−1 was attributed to the external vibration of BiVO4. The peaks at 330.6 cm−1 and 373.1 cm−1 were caused by V–O asymmetrical vibration and V–O bending vibration in the VO43− unit, while the peaks at 717 and 834.0 cm−1 could be attributed to two different modes of stretching vibration of V–O [39,40,41,42]. Ni-BiVO4 samples showed similar Raman peaks with BiVO4, but the band position at 834.0 cm−1 right-shifted gradually with increasing Ni doping concentration (Fig. 1d), indicating the gradual deformation of VO43– [43]. This should be caused by the much smaller atomic radius of doped Ni2+ (0.78 Å) rather than Bi3+ (1.03 Å), leading to the crystal deformation and surface defects.
SEM images of BiOI, pure BiVO4, and Ni-doped BiVO4 samples are shown in Fig. 2a–e. The BiOI crystals, with or without Ni, had similar structures of thin two-dimensional nanoflakes (Fig. 2a, Fig. S1 in Supporting Information), which was beneficial for V precursor diffusion to produce BiVO4. However, the Ni doping made BiOI structures much denser (Fig. S1), benefiting the crystal size decrease in the produced BiVO4 [18]. After thermal calcination, all samples possessed the sponge-like network film with a thickness of ca. 1 μm (Fig. 2b–e). And, compared with pure BiVO4, Ni doping decreased the size of BiVO4 nanoparticles, and higher Ni content led to smaller crystal sizes (BiVO4, 500 nm; 3-Ni-BiVO4, 440 nm; 5-Ni-BiVO4, 310 nm; 10-Ni-BiVO4, 200 nm). The contractible particle might have enhanced the separation efficiency of the photo-induced charges [44]. Figure 2f shows the typical nanoparticle stacking structure of doped BiVO4 (5-Ni-BiVO4), which assembled the sponge-like network of BiVO4. From HR-TEM images (Fig. 2g, h), the lattice fringes with intervals of 0.475 nm and 0.292 nm corresponded to the (110) and (040) planes of the BiVO4, respectively. As shown in EDS mapping (Fig. 2i–l), the uniform Ni distribution in the BiVO4 proves that Ni species are successfully doped in the lattice. ICP data (Table S1) indicated that the content of Ni rose gradually from 3-Ni-BiVO4 to 10-Ni-BiVO4.
Chemical State
The chemical states of BiVO4 and Ni-BiVO4 samples were detected by XPS. The binding energies of Bi 4f7/2 at 159 eV and Bi 4f5/2 at 164.3 eV were assigned to Bi3+ species (Fig. 3a), while the V 2p1/2 and V 2p3/2 peaks of BiVO4 located at 523.8 eV and 516.5 eV referred to V5+ species (Fig. 3b). But for Ni-BiVO4, the obvious asymmetric peak of V 2p3/2 confirmed the existence of V4+ species (516.1 eV) (related to oxygen vacancies) [45]. Furthermore, the Bi 4f peaks of Ni-BiVO4 all shifted to lower binding energies compared to pure BiVO4, owing to the lower electronegativities of Ni2+ than Bi3+ via the Bi-O-Ni connection [35, 38]. In addition, the doping of Ni resulted in the presence of oxygen vacancies, which also led to the obvious red shift of Bi 4f [16].
It is worth noting that the concentrations of V4+ species and oxygen vacancies increased with the doping concentration, but stayed constant when the doping concentration was ≥ 5%. From Fig. 3d, the binding energies of the dominant Ni 2p XPS peaks were found to locate at 855.7, 861.3, 873.6 and 879.8 eV, and contributed to the Ni 2p3/2 main peak, Ni 2p1/2 main peak, Ni 2p3/2 satellite peak, and Ni 2p1/2 satellite peak, respectively. The peak intensity strengthened with the amount of Ni doping, confirming the effective Ni doping in the BiVO4 lattice. The decreasing concentration of free electrons in Ni-BiVO4 has been reported to stabilize the oxygen defects [46].
Optical Properties
The optical absorption spectra were analyzed and are shown in Fig. 4a. The absorption band edge of pure BiVO4 is at about 530 nm, corresponding to its bandgap. Importantly, Ni doping led to stronger absorption intensity and a red shift of the absorption edge, which was enhanced gradually with the increase in Ni concentration.
According to the absorption spectrum, the band gap of samples was calculated as follows:
where α, hν, A, Eg, and n are the absorption coefficient, photon energy, proportionality constant, band gap energy, and a coefficient, respectively. Since BiVO4 was an indirect band gap material, the n value was 2.
As shown in Fig. 4a, the band gap of BiVO4 was ca. 2.43 eV, while those of Ni-doped BiVO4 were 2.42 eV for 3-Ni-BiVO4, 2.39 eV for 5-Ni-BiVO4, and 2.38 eV for 10-Ni-BiVO4. The decrease in band gap should be attributed to the introduced band levels of Ni-doping-mediated V4+ species and oxygen defects.
Photoluminescence (PL) spectra were performed to investigate the charge carrier recombination. As shown in Fig. 4b, the main emission peaks of all samples were at ca. 530 nm, for the recombination of extrinsic radiative transitions [16]. Notably, Ni-doped BiVO4 samples exhibited a lower emission peak intensity than BiVO4, suggesting that the recombination of charge pairs was inhibited and more holes transferred to the surface to trigger the water oxidation reaction [47]. However, too many dopants (10-Ni-BiVO4) lead to an increase in emission peak intensity, attributed to the fact that part of the foreign Ni species acts at the recombination sites and suppresses the PEC performance [33].
PEC Performance
PEC performance of the samples was examined under AM 1.5 G (100 mW/cm2), and the results are shown in Fig. 5a. Under a dark environment, no photocurrent could be observed for BiVO4, while it showed a photocurrent of 0.94 mA/cm2 at 1.23 V versus RHE under light irradiation. Importantly, the Ni doping could significantly improve the PEC performance, i.e., 1.91 mA/cm2 (1.23 V vs. RHE) for 3-Ni-BiVO4. Particularly, 5-Ni-BiVO4 showed the highest photocurrent of 2.39 mA/cm2 (1.23 V vs. RHE), which was 2.5 times higher than pure BiVO4. However, further increasing the Ni doping amount resulted in a decrease in PEC performance, i.e., 1.73 mA/cm2 for 10-Ni-BiVO4, because the excess of Ni species may serve as the recombination center of photogenerated charges and inhibit the PEC activity [33]. Meanwhile, the incident photon-to-current conversion efficiency (IPCE) was determined (Fig. 5b), and Ni doping could obviously promote the IPCE value. In particular, the IPCE value of 5-Ni-BiVO4 was up to 45% at 400–450 nm, while it was only 25% for pure BiVO4. Similarly, the applied bias photon-to-current efficiency (ABPE) was tested to be 0.55% for 5-Ni-BiVO4 at 0.8 V versus RHE, while only 0.19% for pure BiVO4 (Fig. S2). In addition, 5-Ni-BiVO4 showed good photostability (Fig. 5c).
In electrochemical impedance spectra (EIS), the arc radius of Nyquist plots can be used to evaluate the charge transfer resistance (Rct) at the semiconductor/electrolyte interface, and the smaller arc radius implies lower Rct. The Rct was calculated based on the fitting model, as seen in the inset of Fig. 5d, and the data are shown in Table S1. The Rct was in the order of Rct(5-Ni-BiVO4) < Rct(3-Ni-BiVO4) < Rct(10-Ni-BiVO4) < Rct(BiVO4). Obviously, the conductivity of BiVO4 was significantly decreased by Ni doping and surface defects, and 5-Ni-BiVO4 showed the lowest resistivity, consistent with the PEC performances.
Therefore, the optimal Ni doping (5-Ni-BiVO4) can decrease the BiVO4 particle size and form abundant surface V4+ and oxygen defects, which then introduces new band levels in the band gap and accelerates the charge transfer [16], benefiting optical absorption, charge separation efficiency, and finally the PEC performance.
Conclusions
We synthesized Ni-doped BiVO4 through an in situ electrodeposition method. The doping can decrease the particle size of BiVO4, and lead to the formation of V4+ species and oxygen defects. Accordingly, the doped BiVO4 shows high optical absorption and rapid charge transfer. Therefore, Ni-doped BiVO4 shows a much higher PEC activity than the pure form. Specifically, 5-Ni-BiVO4 exhibits the best PEC performance, with the photocurrent as high as 2.39 mA/cm2 at 1.23 V versus RHE, a high IPCE (400 nm) value of 45%, and good PEC stability.
References
Ji LL, Lv CC, Chen ZF et al (2018) Nickel-based (photo) electrocatalysts for hydrogen production. Adv Mater 30:e1705653
Wang YF, Zhang ZY, Shang QQ et al (2018) Synthesis and optimization of TiO2/graphene with exposed 001 facets based on response surface methodology and evaluation of enhanced photocatalytic activity. Trans Tianjin Univ 24:415–423
Wang YF, Zou YL, Shang QQ et al (2018) Quasi-spherical brookite TiO2 nanostructures synthesized using solvothermal method in the presence of oxalic acid. Trans Tianjin Univ 24:326–339
Tan X, Li XL, Yu T et al (2016) Preparation and photocatalytic activity of BiOBr/TiO2 heterojunction nanocomposites. Trans Tianjin Univ 22:211–217
Kim JH, Jo Y, Kim JH et al (2015) Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9:11820–11829
Fujishima A, Honda K (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38
Walter MG, Warren EL, McKone JR et al (2010) Solar water splitting cells. Chem Rev 110:6446–6473
Wang ZL, Lv JL, Zhang JL et al (2018) Facile synthesis of Z-scheme BiVO4/porous graphite carbon nitride heterojunction for enhanced visible-light-driven photocatalyst. Appl Surf Sci 430:595–602
Tee SY, Win KY, Teo WS et al (2017) Recent progress in energy-driven water splitting. Adv Sci 4:1600337
Kang D, Kim TW, Kubota SR et al (2015) Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem Rev 115:12839–12887
Huang ZF, Pan L, Zou JJ et al (2014) Nanostructured bismuth vanadate-based materials for solar-energy-driven water oxidation: a review on recent progress. Nanoscale 6:14044–14063
Park Y, Mcdonald KJ, Choi KS (2013) Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem Soc Rev 42:2321–2337
Tang YQ, Wang RR, Yang Y et al (2016) Highly enhanced photoelectrochemical water oxidation efficiency based on triadic quantum dot/layered double hydroxide/BiVO4 photoanodes. ACS Appl Mater Interfaces 8:19446–19455
Chen F, Yang Q, Sun J et al (2016) Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: mineralization efficiency and mechanism. ACS Appl Mater Interfaces 8:32887–32900
Qiu YC, Liu W, Chen W et al (2016) Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells. Sci Adv 2:e1501764
Wu JM, Chen Y, Pan L et al (2018) Multi-layer monoclinic BiVO4 with oxygen vacancies and V4+ species for highly efficient visible-light photoelectrochemical applications. Appl Catal B 221:187–195
Saito R, Miseki Y, Sayama K (2012) Highly efficient photoelectrochemical water splitting using a thin film photoanode of BiVO4/SnO2/WO3 multi-composite in a carbonate electrolyte. Chem Commun 48:3833–3835
Mcdonald KJ, Choi KS (2012) A new electrochemical synthesis route for a BiOI electrode and its conversion to a highly efficient porous BiVO4 photoanode for solar water oxidation. Energy Environ Sci 9:8553–8557
Long MC, Cai WM, Kisch H (2008) Visible light induced photoelectrochemical properties of n-BiVO4 and n-BiVO4/p-Co3O4. J Phys Chem C 112:548–554
Lee MG, Kim DH, Sohn W et al (2016) Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation. Nano Energy 28:250–260
Seabold JA, Choi KS (2012) Efficient and stable photo-oxidation of water by a bismuth vanadate photoanode coupled with an iron oxyhydroxide oxygen evolution catalyst. J Am Chem Soc 134:2186–2192
Huang MY, Bian JC, Xiong W et al (2018) Low-dimensional Mo: BiVO4 photoanodes for enhanced photoelectrochemical activity. J Mater Chem A 6:3602–3609
Yoon KR, Ko JW, Youn DY et al (2016) Synthesis of Ni-based co-catalyst functionalized W: BiVO4 nanofibers for solar water oxidation. Green Chem 18:944–950
Nair V, Perkins CL, Lin QY et al (2016) Textured nanoporous Mo: BiVO4 photoanodes with high charge transport and charge transfer quantum efficiencies for oxygen evolution. Energy Environ Sci 9:1412–1429
Jovic V, Laverock J, Rettie AJE et al (2015) Soft X-ray spectroscopic studies of the electronic structure of M: BiVO4 (M = Mo, W) single crystals. J Mater Chem A 3:23743–23753
Pilli SK, Furtak TE, Brown LD et al (2011) Cobalt-phosphate (Co-Pi) catalyst modified Mo-doped BiVO4 photoelectrodes for solar water oxidation. Energy Environ Sci 4:5028–5034
Yao W, Iwai H, Ye J (2008) Effects of molybdenum substitution on the photocatalytic behavior of BiVO4. Dalton Trans 21:1426–1430
Luo WJ, Yang ZS, Li ZS et al (2011) Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ Sci 4:4046–4051
Zhong DK, Choi S, Gamelin DR (2011) Near-complete suppression of surface recombination in solar photoelectrolysis by “Co–Pi” catalyst-modified W: BiVO4. J Am Chem Soc 133:18370–18377
Ye H, Lee J, Jang JS et al (2010) Rapid screening of BiVO4-based photocatalysts by scanning electrochemical microscopy (SECM) and studies of their photoelectrochemical properties. J Phys Chem C 114:13322–13328
Ye H, Park HS, Bard AJ (2011) Screening of electrocatalysts for photoelectrochemical water oxidation on W-doped BiVO4 photocatalysts by scanning electrochemical microscopy. J Phys Chem C 115:12464–12470
Gao B, Wang T, Fan XL et al (2017) Synthesis of yellow mesoporous Ni-doped TiO2 with enhanced photoelectrochemical performance under visible light. Inorg Chem Front 4:898–906
Li J, Wang J, Zhang GK et al (2018) Enhanced molecular oxygen activation of Ni2+-doped BiO2−x nanosheets under UV, visible and near-infrared irradiation: mechanism and DFT study. Appl Catal B 234:167–177
Zhou B, Zhao X, Liu HJ et al (2011) Synthesis of visible-light sensitive M-BiVO4 (M = Ag Co, and Ni) for the photocatalytic degradation of organic pollutants. Sep Purif Technol 77(3):275–282
Meng WW, Wang L, Li YH et al (2018) Enhanced sensing performance of mixed potential ammonia gas sensor based on Bi0.95Ni0.05VO3.975 by silver. Sensors Actuator B Chem 259:668–676
Quiñonero J, Gómez R (2017) Controlling the amount of co-catalyst as a critical factor in determining the efficiency of photoelectrodes: the case of nickel (II) hydroxide on vanadate photoanodes. Appl Catal B 217:437–447
Kim TW, Choi KS (2014) Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343:990–994
Park HS, Kweon KE, Ye H et al (2011) Factors in the metal doping of BiVO4 for improved photoelectrocatalytic activity as studied by scanning electrochemical microscopy and first-principles density-functional calculation. J Phys Chem C 115:17870–17879
Madhusudan P, Ran JR, Zhang J et al (2011) Novel urea assisted hydrothermal synthesis of hierarchical BiVO4/Bi2O2CO3 nanocomposites with enhanced visible-light photocatalytic activity. Appl Catal B 110:286–295
Li GS, Zhang DQ, Yu JC (2008) Ordered mesoporous BiVO4 through nanocasting: a superior visible light-driven photocatalyst. Chem Mater 20:3983–3992
Yu JQ, Kudo A (2006) Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv Funct Mater 16:2163–2169
Frost RL, Henry DA, Weier ML et al (2006) Raman spectroscopy of three polymorphs of BiVO4: clinobisvanite, dreyerite and pucherite, with comparisons to (VO4)3− bearing minerals: Namibite, pottsite and schumacherite. J Raman Spectrosc 37:722–732
Karunakaran C, Kalaivani S (2015) Synthesis of nanoparticulate in-doped BiVO4 for enhanced visible-light photocatalytic degradation of dye. Int J Appl Ceram Tec 12:711–721
Luo B, Liu G, Wang LZ (2016) Recent advances in 2D materials for photocatalysis. Nanoscale 8:6904–6920
Wang SC, Chen P, Yun JH et al (2017) An electrochemically treated BiVO4 photoanode for efficient photoelectrochemical water splitting. Angew Chem Int Ed Engl 56:8500–8504
Wu QP, Vandekrol R (2012) Selective photoreduction of nitric oxide to nitrogen by nanostructured TiO2 photocatalysts: role of oxygen vacancies and iron dopant. J Am Chem Soc 134:9369–9375
Zhou FQ, Min YL, Fan JC et al (2015) Reduced graphene oxide-grafted cylindrical like W doped BiVO4 hybrids with enhanced performances for photocatalytic applications. Chem Eng J 266:48–55
Acknowledgements
The authors are grateful for the support from the National Natural Science Foundation of China (Nos. 51661145026, 21506156, 21676193) and the Tianjin Municipal Natural Science Foundation (No. 16JCQNJC05200).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kong, D., Qi, J., Liu, D. et al. Ni-Doped BiVO4 with V4+ Species and Oxygen Vacancies for Efficient Photoelectrochemical Water Splitting. Trans. Tianjin Univ. 25, 340–347 (2019). https://doi.org/10.1007/s12209-019-00202-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12209-019-00202-1