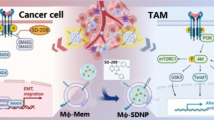
Abstract
Introduction
Treatment options for cancer metastases, the primary cause of cancer mortality, are limited. The chemokine receptor CXCR4 is an attractive therapeutic target in cancer because it mediates metastasis by inducing cancer cell and macrophage migration. Here we engineered carrier-free CXCR4-targeting RNA-protein nanoplexes that not only inhibited cellular migration but also polarized macrophages to the M1 phenotype.
Materials and Methods
A CXCR4-targeting single-chain variable fragment (scFv) antibody was fused to a 3030 Da RNA-binding protamine peptide (RSQSRSRYYRQRQRSRRRRRRS). Self-assembling nanoplexes were formed by mixing the CXCR4-scFv-protamine fusion protein (CXCR4-scFv-RBM) with miR-127-5p, a miRNA shown to mediate M1 macrophage polarization. RNA-protein nanoplexes were characterized with regard to their physicochemical properties and therapeutic efficacy.
Results
CXCR4-targeting RNA-protein nanoplexes simultaneously acted as a targeting ligand, a macrophage polarizing drug, and a miRNA delivery vehicle. Our carrier-free, RNA-protein nanoplexes specifically bound to CXCR4-positive macrophages and breast cancer cells, showed high drug loading (~ 90% w/w), and are non-toxic. Further, these RNA-protein nanoplexes significantly inhibited cancer and immune cell migration (75 to 99%), robustly polarized macrophages to the tumor-suppressive M1 phenotype, and inhibited tumor growth in a mouse model of triple-negative breast cancer
Conclusions
We engineered a novel class of non-toxic RNA-protein nanoplexes that modulate the tumor stroma. These nanoplexes are promising candidates for add-ons to clinically approved chemotherapeutics.









Similar content being viewed by others
Abbreviations
- scFv:
-
Single-chain variable fragment
- RBM:
-
RNA-binding motif
References
Aras, S., and M. R. Zaidi. TAMeless traitors: macrophages in cancer progression and metastasis. Br. J. Cancer 117:1583–1591, 2017.
Brown, J. M., L. Recht, and S. Strober. The promise of targeting macrophages in cancer therapy. Clin. Cancer Res. 23:3241–3250, 2017.
Burger, J. A., and T. J. Kipps. CXCR18: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 107:1761–1767, 2006.
Chatterjee, S., B. Behnam Azad, and S. Nimmagadda. The intricate role of CXCR1 in cancer. Adv. Cancer Res. 124:31–82, 2014.
Chen, Y., S. Zhang, Q. Wang, and X. Zhang. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J. Hematol. Oncol. 10:36, 2017.
Cheok, C. F. Protecting normal cells from the cytotoxicity of chemotherapy. Cell Cycle 11:2227–2228, 2012.
Chu, K. S., A. N. Schorzman, M. C. Finniss, C. J. Bowerman, L. Peng, J. C. Luft, A. J. Madden, A. Z. Wang, W. C. Zamboni, and J. M. DeSimone. Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials 34:8424–8429, 2013.
Darash-Yahana, M., E. Pikarsky, R. Abramovitch, E. Zeira, B. Pal, R. Karplus, K. Beider, S. Avniel, S. Kasem, E. Galun, and A. Peled. Role of high expression levels of CXCR26 in tumor growth, vascularization, and metastasis. FASEB J. 18:1240–1242, 2004.
Deci, M. B., S. W. Ferguson, M. Liu, D. C. Peterson, S. P. Koduvayur, and J. Nguyen. Utilizing clathrin triskelions as carriers for spatially controlled multi-protein display. Biomaterials 108:120–128, 2016.
Deci, M. B., S. W. Ferguson, S. L. Scatigno, and J. Nguyen. Modulating macrophage polarization through CCR7 inhibition and multivalent engagement. Mol. Pharm. 15:2721–2731, 2018.
Deci, M. B., M. Liu, Q. T. Dinh, and J. Nguyen. Precision engineering of targeted nanocarriers. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10:e1511, 2018.
Dong, X., C. A. Mattingly, M. T. Tseng, M. J. Cho, Y. Liu, V. R. Adams, and R. J. Mumper. Doxorubicin and paclitaxel-loaded lipid-based nanoparticles overcome multidrug resistance by inhibiting P-glycoprotein and depleting ATP. Cancer Res. 69:3918–3926, 2009.
Ferguson, S., S. Kim, C. Lee, M. Deci, and J. Nguyen. The phenotypic effects of exosomes secreted from distinct cellular sources: a comparative study based on miRNA composition. AAPS J. 20:67, 2018.
Furusato, B., A. Mohamed, M. Uhlen, and J. S. Rhim. CXCR3 and cancer. Pathol. Int. 60:497–505, 2010.
Ganapathy, V., P. V. Moghe, and C. M. Roth. Targeting tumor metastases: drug delivery mechanisms and technologies. J. Control. Release 219:215–223, 2015.
Haidaris, C. G., J. Malone, L. A. Sherrill, J. M. Bliss, A. A. Gaspari, R. A. Insel, and M. A. Sullivan. Recombinant human antibody single chain variable fragments reactive with Candida albicans surface antigens. J. Immunol. Methods 257:185–202, 2001.
Hu, H., J.-J. Hang, T. Han, M. Zhuo, F. Jiao, and L.-W. Wang. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumor Biol. 37:8657–8664, 2016.
Huang, R., K. T. Gorman, C. R. Vinci, E. Dobrovetsky, S. Graslund, and B. K. Kay. Streamlining the pipeline for generation of recombinant affinity reagents by integrating the affinity maturation step. Int. J. Mol. Sci. 16:23587–23603, 2015.
Hughes, R., B. Z. Qian, C. Rowan, M. Muthana, I. Keklikoglou, O. C. Olson, S. Tazzyman, S. Danson, C. Addison, M. Clemons, A. M. Gonzalez-Angulo, J. A. Joyce, M. De Palma, J. W. Pollard, and C. E. Lewis. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res. 75:3479–3491, 2015.
Italiani, P., and D. Boraschi. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front. Immunol. 5:514, 2014.
Jensen, J. L., A. Rakhmilevich, E. Heninger, A. T. Broman, C. Hope, F. Phan, S. Miyamoto, I. Maroulakou, N. Callander, P. Hematti, M. Chesi, P. L. Bergsagel, P. Sondel, and F. Asimakopoulos. Tumoricidal effects of macrophage-activating immunotherapy in a murine model of relapsed/refractory multiple myeloma. Cancer Immunol. Res. 3:881–890, 2015.
Li, H., X. Fan, and J. Houghton. Tumor microenvironment: the role of the tumor stroma in cancer. J. Cell. Biochem. 101:805–815, 2007.
Nguyen, J., R. Reul, S. Roesler, E. Dayyoub, T. Schmehl, T. Gessler, W. Seeger, and T. H. Kissel. Amine-modified poly(vinyl alcohol)s as non-viral vectors for siRNA delivery: effects of the degree of amine substitution on physicochemical properties and knockdown efficiency. Pharm. Res. 27:2670–2682, 2010.
Nguyen, J., T. W. Steele, O. Merkel, R. Reul, and T. Kissel. Fast degrading polyesters as siRNA nano-carriers for pulmonary gene therapy. J. Control. Release 132:243–251, 2008.
Nguyen, J., C. L. Walsh, J. P. Motion, E. K. Perttu, and F. Szoka. Controlled nucleation of lipid nanoparticles. Pharm. Res. 29:2236–2248, 2012.
Rana, K., J. L. Liesveld, and M. R. King. Delivery of apoptotic signal to rolling cancer cells: A novel biomimetic technique using immobilized TRAIL and E-selectin. Biotechnol. Bioeng. 102:1692–1702, 2009.
Reul, R., J. Nguyen, A. Biela, E. Marxer, U. Bakowsky, G. Klebe, and T. Kissel. Biophysical and biological investigation of DNA nano-complexes with a non-toxic, biodegradable amine-modified hyperbranched polyester. Int. J. Pharm. 436:97–105, 2012.
Sanchez-Martin, L., A. Estecha, R. Samaniego, S. Sanchez-Ramon, M. A. Vega, and P. Sanchez-Mateos. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117:88–97, 2011.
Seyfried, T. N., and L. C. Huysentruyt. On the origin of cancer metastasis. Crit. Rev. Oncog. 18:43–73, 2013.
Tardi, P. G., R. C. Gallagher, S. Johnstone, N. Harasym, M. Webb, M. B. Bally, and L. D. Mayer. Coencapsulation of irinotecan and floxuridine into low cholesterol-containing liposomes that coordinate drug release in vivo. Biochim. Biophys. Acta (BBA): Biomembranes 1768:678–687, 2007.
Wynn, T. A., and K. M. Vannella. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44:450–462, 2016.
Yao, Y. D., T. M. Sun, S. Y. Huang, S. Dou, L. Lin, J. N. Chen, J. B. Ruan, C. Q. Mao, F. Y. Yu, M. S. Zeng, J. Y. Zang, Q. Liu, F. X. Su, P. Zhang, J. Lieberman, J. Wang, and E. Song. Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. Sci. Transl. Med. 4:130ra48, 2012.
Ying, H., Y. Kang, H. Zhang, D. Zhao, J. Xia, Z. Lu, H. Wang, F. Xu, and L. Shi. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J. Immunol. 194:1239–1251, 2015.
Zhang, X., R. Goncalves, and D. M. Mosser. The isolation and characterization of murine macrophages, Chapter 14:Unit 14 1. Curr. Protoc. Immunol. 2008. https://doi.org/10.1002/0471142735.im1401s83.
Acknowledgments
We acknowledge support from the NIH through Awards EB023262 and EB021454. We thank Dr. Mark Sullivan for providing us with the phage display library. We thank Dr. Brian Kay and Dr. Renhua Huang for training in techniques related to phage display and screening. We thank Dr. Gauri Rao for helpful discussions.
Conflict of interest
All authors, including M.B. Deci, M. Liu, J. Gonya, C.J. Lee, Tingyi Li, S.W. Ferguson, E.E. Bonacquisti, J. Wang, and J. Nguyen, declare that they have no conflicts of interest.
Ethical Approval
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. No human studies were carried out by the authors for this article
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Michael King oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juliane Nguyen is an Associate Professor in the Division of Pharmacoengineering and Molecular Therapeutics at the University of North Carolina at Chapel Hill. She received her PharmD and PhD in Pharmaceutical Sciences from the Philipps-University of Marburg (2009), where she trained with Professor Thomas Kissel. From 2009 to 2013, she was a Deutsche Forschungsgemeinschaft postdoctoral fellow in Professor Francis Szoka’s laboratory at the University of California, San Francisco. Dr. Nguyen started her laboratory at the University at Buffalo in July 2013 and was promoted to Associate Professor in January 2019. Her laboratory develops novel protein-, RNA-, and lipid-based biochemical and delivery platforms for treating myocardial infarction and cancer. Dr. Nguyen is the recipient of numerous awards, including the Biomedical Breakthrough Award (2011), the University at Buffalo Exceptional Scholar Young Investigator Award (2017), the Pioneering Pharmaceutical Sciences by Emerging Investigator Award (2018), the NSF CAREER Award (2018), and the NYSTAR faculty award (2019).
This article is part of the CMBE 2019 Young Innovators special issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deci, M.B., Liu, M., Gonya, J. et al. Carrier-Free CXCR4-Targeted Nanoplexes Designed for Polarizing Macrophages to Suppress Tumor Growth. Cel. Mol. Bioeng. 12, 375–388 (2019). https://doi.org/10.1007/s12195-019-00589-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-019-00589-w