Abstract
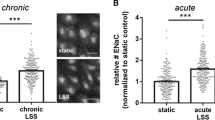
Recent evidence indicates that several experimental pathophysiological conditions are associated with elevated protease activity in plasma, which impacts endothelial function. We hypothesize that extracellular structures bound to the endothelial cell (EC) membrane may be degraded by proteolytic activity reducing the cell’s response to physiological shear stress and glucose metabolism efficiency. To test this hypothesis, cultured bovine aortic endothelial cells (BAECs) were exposed to low serine protease activity. Extracellular mechanosensor densities of the glycocalyx and vascular endothelial growth factor receptor 2 (VEGFR-2) were determined. Metabolic dysfunction was tested by examining insulin receptor and glucose uptake levels. Protease treatment impaired the cells’ ability to align in the direction of fluid flow after 12 h of shear stress (12 dyn/cm2); however, cells realigned after an additional 12 h of shear stress with protease inhibition. Proteases caused reduction in the densities of glycocalyx, VEGFR-2, and insulin receptor in static and shear conditions, except for static VEGFR-2 cells. Under static conditions, protease-treated ECs had reduced glucose uptake compared to untreated controls. Under shear, glucose uptake for protease treated BAECs was greater than untreated controls. In conclusion, protease activity in plasma alters the exofacial membrane components of ECs and may interfere with mechanotransduction.





Similar content being viewed by others
References
Arisaka, T., M. Mitsumata, M. Kawasumi, T. Tohjima, S. Hirose, and Y. Yoshida. Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann. N. Y. Acad. Sci. 748:543–554, 1995.
Blanquart, C., J. Achi, and T. Issad. Characterization of IRA/IRB hybrid insulin receptors using bioluminescence resonance energy transfer. Biochem. Pharmacol. 76(7):873–883, 2008.
Chen, A. Y., F. A. DeLano, S. R. Valdez, J. N. Ha, H. Y. Shin, and G. W. Schmid-Schönbein. Receptor cleavage reduces the fluid shear response in neutrophils of the spontaneously hypertensive rat. Am. J. Physiol. Cell Physiol. 299(6):C1441–C1449, 2010.
Chen, B. P., Y. S. Li, Y. Zhao, K. D. Chen, S. Li, J. Lao, S. Yuan, J. Y. Shyy, and S. Chien. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol. Genomics 7(1):55–63, 2001.
Cho, C. H., C. S. Lee, M. Chang, I. H. Jang, S. J. Kim, I. Hwang, S. H. Ryu, C. O. Lee, and G. Y. Koh. Localization of VEGFR-2 and PLD2 in endothelial caveolae is involved in VEGF-induced phosphorylation of MEK and ERK. Am. J. Physiol. Heart Circ. Physiol. 286(5):H1881–H1888, 2004.
DeLano, F. A., and G. W. Schmid-Schönbein. Proteinase activity and receptor cleavage: mechanism for insulin resistance in the spontaneously hypertensive rat. Hypertension 52(2):415–423, 2008.
DeLano, F. A., and G. W. Schmid-Schönbein. Proteolytic cleavage of insulin receptor by pancreatic digestive enzymes leads to insulin resistance in hemorrhagic shock. FASEB J. 24:788.7, 2010.
Delano, F. A., H. Zhang, E. E. Tran, C. Zhang, and G. W. Schmid-Schönbein. A new hypothesis for insulin resistance in hypertension due to receptor cleavage. Expert Rev. Endocrinol. Metab. 5(1):149–158, 2010.
Dugani, C. B., V. K. Randhawa, A. W. Cheng, N. Patel, and A. Klip. Selective regulation of the perinuclear distribution of glucose transporter 4 (GLUT4) by insulin signals in muscle cells. Eur. J. Cell Biol. 87(6):337–351, 2008.
Fahy, B. G., A. M. Sheehy, and D. B. Coursin. Glucose control in the intensive care unit. Crit. Care Med. 37(5):1769–1776, 2009.
Gao, L., and H. H. Lipowsky. Measurement of solute transport in the endothelial glycocalyx using indicator dilution techniques. Ann. Biomed. Eng. 37(9):1781–1795, 2009.
Holmes, K., O. L. Roberts, A. M. Thomas, and M. J. Cross. Vascular endothelial growth factor receptor-2: structure, function, intracellular signalling and therapeutic inhibition. Cell. Signal. 19(10):2003–2012, 2007.
Hou, X., M. A. Weiler, J. N. Winger, J. R. Morris, and J. L. Borke. Rat model for studying tissue changes induced by the mechanical environment surrounding loaded titanium implants. Int. J. Oral Maxillofac. Implants 24(5):800–807, 2009.
Masini, E., S. Cuzzocrea, E. Mazzon, C. Muia, A. Vannacci, F. Fabrizi, and D. Bani. Protective effects of relaxin in ischemia/reperfusion-induced intestinal injury due to splanchnic artery occlusion. Br. J. Pharmacol. 148(8):1124–1132, 2006.
Matsuda, N., H. Teramae, S. Yamamoto, K. Takano, Y. Takano, and Y. Hattori. Increased death receptor pathway of apoptotic signaling in septic mouse aorta: effect of systemic delivery of FADD siRNA. Am. J. Physiol. Heart Circ. Physiol. 298(1):H92–H101, 2010.
Merks, R. M., E. D. Perryn, A. Shirinifard, and J. A. Glazier. Contact-inhibited chemotaxis in de novo and sprouting blood-vessel growth. PLoS Comput. Biol. 4(9):e1000163, 2008.
Olsen, J. V., S. E. Ong, and M. Mann. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteomics 3(6):608–614, 2004.
Pace, C. N., and A. J. Barrett. Kinetics of tryptic hydrolysis of the arginine-valine bond in folded and unfolded ribonuclease T1. Biochem. J. 219(2):411–417, 1984.
Paczek, L., W. Michalska, and I. Bartlomiejczyk. Proteolytic enzyme activity as a result of aging. Aging Clin. Exp. Res. 21(1):9–13, 2009.
Penn, A. H., T. E. Hugli, and G. W. Schmid-Schönbein. Pancreatic enzymes generate cytotoxic mediators in the intestine. Shock 27(3):296–304, 2007.
Rosario, H. S., S. W. Waldo, S. A. Becker, and G. W. Schmid-Schönbein. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am. J. Pathol. 164(5):1707–1716, 2004.
Schmid-Schönbein, G. W. 2008 Landis award lecture. Inflammation and the autodigestion hypothesis. Microcirculation 16(4):289–306, 2009.
Shay-Salit, A., M. Shushy, E. Wolfovitz, H. Yahav, F. Breviario, E. Dejana, and N. Resnick. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. USA 99(14):9462–9467, 2002.
Shibuya, M. Vascular endothelial growth factor receptor family genes: when did the three genes phylogenetically segregate? Biol. Chem. 383(10):1573–1579, 2002.
Somanath, P. R., N. L. Malinin, and T. V. Byzova. Cooperation between integrin alphavbeta3 and VEGFR2 in angiogenesis. Angiogenesis 12(2):177–185, 2009.
Squire, J. M., M. Chew, G. Nneji, C. Neal, J. Barry, and C. Michel. Quasi-periodic substructure in the microvessel endothelial glycocalyx: a possible explanation for molecular filtering? J. Struct. Biol. 136(3):239–255, 2001.
Stucky, C. L., and G. R. Lewin. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. J. Neurosci. 19(15):6497–6505, 1999.
Suematsu, M., F. A. DeLano, D. Poole, R. L. Engler, M. Miyasaka, B. W. Zweifach, and G. W. Schmid-Schönbein. Spatial and temporal correlation between leukocyte behavior and cell injury in postischemic rat skeletal muscle microcirculation. Lab. Invest. 70(5):684–695, 1994.
Sun, R. J., S. Muller, J. F. Stoltz, and X. Wang. Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur. Biophys. J. 30(8):605–611, 2002.
Sun, Z., X. Wang, A. Lasson, A. Bojesson, M. Annborn, and R. Andersson. Effects of inhibition of PAF, ICAM-1 and PECAM-1 on gut barrier failure caused by intestinal ischemia and reperfusion. Scand. J. Gastroenterol. 36(1):55–65, 2001.
Tarbell, J. M., and E. E. Ebong. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci. Signal 1(40):pt8, 2008.
Tarbell, J. M., S. Weinbaum, and R. D. Kamm. Cellular fluid mechanics and mechanotransduction. Ann. Biomed. Eng. 33(12):1719–1723, 2005.
Tran, E. D., F. A. Delano, and G. W. Schmid-Schönbein. Enhanced matrix metalloproteinase activity in the spontaneously hypertensive rat: VEGFR-2 cleavage, endothelial apoptosis, and capillary rarefaction. J. Vasc. Res. 47(5):423–431, 2010.
Waldo, S. W., H. S. Rosario, A. H. Penn, and G. W. Schmid-Schönbein. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock 20(2):138–143, 2003.
Wang, H., Z. Liu, G. Li, and E. J. Barrett. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 291(2):E323–E332, 2006.
Wang, Z., C. Tiruppathi, R. D. Minshall, and A. B. Malik. Size and dynamics of caveolae studied using nanoparticles in living endothelial cells. ACS Nano 3(12):4110–4116, 2009.
Xu, F., X. Yang, Z. Lu, and F. Kuang. Evaluation of glucose metabolic disorder: insulin resistance and insulin receptors in critically ill children. Chin. Med. J. (Engl.) 109(10):807–809, 1996.
Yamamoto, K., T. Sokabe, T. Watabe, K. Miyazono, J. K. Yamashita, S. Obi, N. Ohura, A. Matsushita, A. Kamiya, and J. Ando. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am. J. Physiol. Heart Circ. Physiol. 288(4):H1915–H1924, 2005.
Yao, Y., A. Rabodzey, and C. F. Dewey, Jr. Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am. J. Physiol. Heart Circ. Physiol. 293(2):H1023–H1030, 2007.
Zhai, L., and J. L. Messina. Age and tissue specific differences in the development of acute insulin resistance following injury. J. Endocrinol. 203(3):365–374, 2009.
Zhuo, L., V. C. Hascall, and K. Kimata. Inter-alpha-trypsin inhibitor, a covalent protein–glycosaminoglycan–protein complex. J. Biol. Chem. 279(37):38079–38082, 2004.
Acknowledgments
We thank Jerry Norwich for assistance with the confocal microscopy. We also would like to thank Dr. Julie Li and Suli Yuan for valuable discussions. This work was supported by grants HL 10881 and GM-85072 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Cheng Dong oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Altshuler, A.E., Morgan, M.J., Chien, S. et al. Proteolytic Activity Attenuates the Response of Endothelial Cells to Fluid Shear Stress. Cel. Mol. Bioeng. 5, 82–91 (2012). https://doi.org/10.1007/s12195-011-0207-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12195-011-0207-6