Abstract
Human herpesvirus type 8 (HHV8) is a gamma herpesvirus known for its role in lymphoid neoplasms, especially in immunosuppressed patients. We describe the case of a 64-year-old male, without known immunodeficiency, with 1-year-long clinical history of mediastinal and abdominal lymphadenopathies and recurrent pulmonary infections. Histopathological evaluation of a mediastinal lymph node revealed the presence of scattered atypical large cells with Hodgkin and Reed–Sternberg morphology in a background of lymphocytes and extensive areas of fibrosis. The large cells were positive for HHV8 and Epstein–Barr virus (EBV), with a clonal pattern of IGH gene rearrangement. A descriptive diagnosis of “HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma” was rendered. Interestingly, the retrospective evaluation of a previous biopsy, diagnosed as reactive lymphadenitis, revealed the presence of HHV8- and EBV-positive cells, with a polyclonal pattern and a small peak corresponding to that of the most recent biopsy. This case presents diagnostic challenges due to the presence of particular features not clearly related to current HHV8-associated entities, and also suggests the possibility for disease progression in the spectrum of HHV8- and EBV-associated lymphoproliferative disorders.
Similar content being viewed by others
Introduction
Human herpesvirus type 8 (HHV8) is a gamma herpesvirus known for its role in the development of varied lymphoid neoplasms, such as primary effusion lymphoma (PEL), multicentric Castleman disease (MCD), germinotropic lymphoproliferative disorder (GLPD) and HHV8-positive diffuse large B-cell lymphoma, not otherwise specified [1,2,3,4]. In addition to these well-characterized HHV8-related entities, occasional cases with atypical and overlapping features, coinfection with Epstein–Barr virus (EBV) and also HHV8-related reactive lymphadenitis have been reported [5,6,7,8,9]. However, the clinical implications of these cases are largely unknown. Moreover, they represent diagnostic and therapeutic challenges as most HHV8-positive lymphoproliferative disorders (LPD) present in the setting of immunosuppression. Here, we report the case of a HHV8- and EBV-associated LPD occurring in an apparently immunocompetent patient, who initially presented with a lesion that was interpreted as “reactive lymphoid hyperplasia”. One year later, the patient developed a large B-cell lymphoma with concurrent HHV8 and EBV infection.
Case report
The patient was a 64-year-old male with a past medical history of a remote thalamic stroke and chronic renal failure without hemodialysis treatment. In March 2016, he was diagnosed with left basal pneumonia being treated with levofloxacin. Laboratory tests included HIV serology negative, Hb: 14.3 g/dL, HCT: 43.7%, leukocytes: 15.76 × 109/L, platelets: 171 × 109/L, erythrocyte sedimentation rate (ESR): 28 mm/h, C-reactive protein (CRP): 1.90 mg/dL, BUN: 52 mg/dL, creatinine: 1.3 mg/dL and glomerular filtration rate (GFR): 59 mL/min/1.73.
The patient did not have history of organ transplant or immunosuppressive treatments. CT imaging showed signs of pneumonia and multiple mediastinal lymphadenopathies. Hence, LPD was suspected and a mediastinoscopy for a lymph node biopsy was performed. We received for histopathological examination three millimetric fragments of lymphoid tissue composed of small lymphoid cells with occasional large cells, which seemed to represent histiocytes and immunoblasts intermingled with reactive-appearing blood vessels in a sclerotic background (Fig. 1a). Immunohistochemical studies showed mixed small sized CD20-positive B-cells (Fig. 1c) and CD3-positive T-cells (Fig. 1e). CD30 and CD15 were negative in both small and large cells. Flow cytometry study did not show any immunophenotypical aberration in B-cell or T-cell populations. In conclusion, a diagnosis of reactive lymphadenitis was rendered, likely related to the infectious process. The patient’s condition improved after antibiotic treatment.
Histopathological and immunophenotypical features of first mediastinal biopsy of 2016, interpreted as “reactive lymphoid hyperplasia” (left column) and second mediastinal biopsy of 2017 diagnosed as HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma (right column). Initial histopathological study of the first mediastinal biopsy shows fragments of lymphoid tissue composed mainly of small lymphoid cells and histiocytes intermingled with reactive-appearing blood vessels in a sclerotic background (a right panel, H&E). Retrospective thorough evaluation of at high magnification reveals scarce atypical large cells, some with hyperchromatic nuclei and irregular nuclear contours (black arrow) (a left panel, H&E). Histopathological analysis of the second biopsy shows a diffuse lymphoid proliferation composed of small lymphocytes and scattered large atypical cells, Hodgkin and Reed–Sternberg-like cells (black arrows) with extensive areas of fibrosis (red arrows) (b H&E). Abundant small B-cells are observed in the first biopsy (c CD20) while in the second biopsy, a reduction in small B-cells is observed (d CD20). Atypical large cells are negative for CD20 (black arrows). A moderate amount of T-cells is observed in the first biopsy (e CD3) with an increase in the numbers of T-cells in the second biopsy (f CD3). Atypical large cells are negative for CD3 (black arrows). In both biopsies, atypical cells with infection by HHV8 (g, h LANA1) and EBV (i, j EBER) were identified, with an increase in the numbers of positive cells from first biopsy (black arrows) to the second biopsy. PD-L1 is positive in the occasional large cells in the first biopsy (black arrows) (k left panel, PD-L1) with rare weakly stained PD1-positive small cells (red arrows) (k right panel, PD1). In the second biopsy, many PD-L1-positive atypical large cells were observed (black arrows) (l left panel, PD-L1). Weakly stained PD1-positive cells were identified (red arrows), while large cells were negative (black arrows) (l right panel, PD1). Original magnifications: a (left panel), g, h, i, j: 10×; b (left panel): 4×; a (right panel), b (right panel), c, d, e, f, k, l insets in g, h, i, j: 40×
One year later (March 2017), the patient developed fever, asthenia, and chills. CT scan showed mediastinal, retroperitoneal, and abdominal lymphadenopathies, as well as signs of acute mediastinitis and pneumonia. He was treated with antibiotics. A new mediastinoscopy was performed. The biopsy of the largest lymph node showed a lymph node with architectural effacement by a diffuse lymphoid proliferation composed of small mature lymphocytes and scattered large atypical cells, resembling Hodgkin and Reed–Sternberg cells. Extensive areas of fibrosis were also observed (Fig. 1b). Hence, a diagnosis of classical Hodgkin lymphoma was considered and a panel of immunostainings, including CD30, CD15, PAX5, CD20 (Fig. 1d), and CD79a was performed. However, the large atypical cells were negative for all these markers. Also, T-cell markers CD3 (Fig. 1f), CD5, CD2, and CD7 were negative in the large cells. Interestingly, the neoplastic cells were positive for HHV8 (LANA1) (Fig. 1h) and EBV (in situ RNA hybridization, EBER) (Fig. 1j). EBV Latency and viral replication studies showed an EBV latency pattern I without viral replication (LMP1−, EBNA2−, ZEBRA−) [10].
Subsequently, a more extensive panel of immunohistochemical markers was carried out including CD45, ALK, MUM1, BCL2, BCL6, CD10, CD138, CD38, HLA-DR, CD68, CD21, CD23, IgD, IgM, IgG, EMA, MYC, S100, CKAE1/AE3, and PD-L1. Large cells were positive for PD-L1 (Fig. 1l, left panel), MUM-1, EMA, BCL2, and BCL6. T-cell markers showed an abundant accompanying T-cell population with a moderate amount of PD1-positive small cells (Fig. 1l, right panel). Immunostaining for MYC was negative. Morphologic features suggestive of MCD or GLPD were not identified. No sheets of large cells were observed. CD21 and CD23 highlighted remnants of follicular dendritic cells in relation to the neoplastic cells. Ki67 staining highlighted the large cells. Kappa and lambda light chains were negative in the large cells.
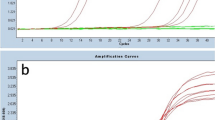
Flow cytometry of the lymph node showed a predominance of T-cells and scarce B-cells, both without immunophenotypical aberrations. To establish the tumor cell lineage, molecular studies of the B-cell and T-cell receptor rearrangements were performed. A monoclonal IGH (FR3) gene rearrangement was identified, while no clonal peak was observed in TCR gamma and beta genes (Fig. 2a). A diagnosis of large HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma was rendered. In addition, B-cell clonality study was done in the first biopsy (year 2016), in which a polyclonal pattern with a small peak corresponding to that of the second biopsy was described (Fig. 2b).
B-cell clonality and FISH studies. IGH gene rearrangement study in the second biopsy (year 2017) diagnosed as HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma shows a monoclonal peak in FR3 (a). In the first biopsy (year 2016), diagnosed initially as reactive lymphoid hyperplasia a polyclonal pattern with a small peak corresponding to that of the second biopsy (year 2017) was observed in the IGH FR3 (b). Composed figure of representative FISH images for PD-L1 in the 2017 biopsy showing atypical large cells with PD-L1 CNAs (PD-L1: orange-red signals/chromosome 9 centromere: green signals) (c). Original magnification: 60×
Since tumor cells expressed PD-L1, a FISH study to detect PD-L1 copy number alterations (CNAs) was performed in the 2017 biopsy, as previously described [10]. Interestingly, we observed disperse atypical large cells with PD-L1 polysomies, gains, and amplifications (Fig. 2c). A thoughtful retrospective histopathological revision of the first biopsy interpreted as reactive lymphoid hyperplasia allowed the identification of scarce HHV8-positive (Fig. 1g) and EBV-positive (Fig. 1i) atypical large cells with PD-L1 expression (Fig. 1k, left panel). In addition, scarce PD1-positive small cells with a weak staining pattern were observed (Fig. 1k, right panel).
The patient was treated with R-COP (Rituximab, Cyclophosphamide, Vincristine, and Prednisolone). However, PET-CT imaging at 5 months after starting chemotherapy showed persistence of lymphadenopathies and severe interstitial lung disease. One month later, he developed a respiratory infection complicated by an acute respiratory distress syndrome, leading to death.
Discussion
Here, we describe a case of HHV8 and EBV-associated LPD occurring in an apparently immunocompetent adult, which has particular features not clearly related to the current HHV8 associated entities (Table 1) [4, 7, 9, 11, 12]. The presence of fibrotic bands and Hodgkin and Reed–Sternberg-like cells in a reactive background initially raised the differential diagnosis of classical Hodgkin’s lymphoma, but the negativity for B-cell markers, CD30, and CD15 ruled out this possibility. Hodgkin’s lymphoma is also consistently negative for HHV8 [13].
The presence of lymphadenopathy in an apparently immunocompetent patient with EBV and HHV8-positive B-cells would suggest GLPD as first differential diagnosis [4]. These cases usually present with a localized nodal enlargement and EBV and HHV8-positive plasmablasts involve preferentially the germinal centers, lack B-cell specific markers and generally have a good prognosis [4]. In contrast, our case presented with multiple lymphadenopathies and no germinotropism could be demonstrated. Moreover, IGH molecular analysis in GLPD usually renders a polyclonal or oligoclonal pattern [4, 7], whereas in the current case an IGH clonal peak was detected and the patient presented a fatal clinical course.
PEL presents as a body cavity effusion in immunosuppressed patients showing an immunoblastic/plasmablastic morphology, and is usually positive for HHV8, EBV, MUM1, IgG, without the expression of B-cell markers, features seen in our case [12]. The extracavitary variant of PEL presents with a mass instead of a body cavity effusion. Nevertheless, the lack of sheets of large cells and the negativity for plasma cell markers CD138 and CD38 do not support this diagnosis [12]. The differential diagnosis also includes large B-cell lymphoma arising in HHV8-associated MCD. However, in these cases, the HHV8-positive plasmablasts are IgM-positive, lambda light chain-restricted, and EBV negative [4].
Previously, a case with comparable clinical, morphological, and immunophenotypical features was reported by Ferry et al. [8]. The patient was an immunocompetent male with cervical and mediastinal lymphadenopathies. The lymph nodes showed dispersed HHV8 and EBV-positive large cells, expressing CD45dim, MUM1, CD138dim, EMA, IgM, with lambda light chain restriction, while CD15, CD30, CD20, and PAX5 were negative [8]. IGH gene rearrangement study showed a polyclonal pattern. The case was similarly interpreted as HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma. The patient received 6 cycles of R-CHOP achieving complete remission, with no evidence of lymphoma 10 months after diagnosis [8].
Hence, HHV8-positive, EBV-positive Hodgkin lymphoma-like large B-cell lymphoma may represent an unrecognized pattern of HHV8 and EBV-associated LPDs. Alternatively, both cases may represent an early lymph nodal involvement by an extracavitary variant of PEL, even though the clinicopathological context does not support this scenario. The presence of recurrent pulmonary infections with a fatal outcome in our case, might suggest the possibility of an under diagnosed immune deficiency, favoring the development of HHV8 and EBV LPDs. Our patient had chronic renal failure, which is associated with cellular immune dysfunction even in the absence of dialysis [14]. This may explain in part the increased susceptibility to pulmonary infections and HHV8 and EBV LPDs with a fatal outcome.
PD-L1/PD1 expression and the presence of PD-L1 CNAs in our case may suggest a role of immunomodulation, which has been associated with EBV in different lymphomas, including Hodgkin lymphoma and LPDs developed in the setting of immunodeficiencies [10, 15]. PD-L1 expression has also been reported in 50% of cases of PEL [15]. However, the presence of PD-L1 CNAs has not been thoroughly studied in HHV8-positive PEL. PD-L1 up-regulation with PD-L1 copy gains may provide the rationale to target the PD1/PD-L1 axis in these cases.
Finally, as we were able to identify in the first biopsy HHV8-positive and EBV-positive cells a pathological progression in the spectrum of HHV8 and EBV-associated LPDs cannot be completely ruled out. In this sense, Guerrero et al., recently reported a case of a HIV-positive patient who sequentially developed several different HHV8-positive LPDs over time [16].
In summary, we describe a HHV8 and EBV-associated LPD whose clinical, morphological, immunohistochemical, and molecular characteristics do not allow classifying it within a specific category recognized by the current WHO classification, expanding the spectrum of LPD with EBV and HHV8 infections.
References
Cathomas G. Kaposi’s sarcoma-associated herpesvirus (KSHV)/human herpesvirus 8 (HHV-8) as a tumour virus. Herpes: J IHMF. 2003;10(3):72–7.
Stebbing J, Pantanowitz L, Dayyani F, Sullivan RJ, Bower M, Dezube BJ. HIV associated multicentric Castleman’s disease. Am J Hematol. 2008;83(6):498–503.
Ariza-Heredia EJ, Razonable RR. Human herpes virus 8 in solid organ transplantation. Transplantation. 2011;92(8):837–44.
Said J, Isaacson PG, Campo E, Harris NL. HHV8-associated lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues (revised 4th edition). Lyon: IARC Press; 2017. p. 325–9.
Courville EL, Sohani AR, Hasserjian RP, Zukerberg LR, Harris NL, Ferry JA. Diverse clinicopathologic features in human herpesvirus 8-associated lymphomas lead to diagnostic problems. Am J Clin Pathol. 2014;142(6):816–29.
Seliem RM, Griffith RC, Harris NL, Beheshti J, Schiffman FJ, Longtine J, et al. HHV-8+, EBV+ multicentric plasmablastic microlymphoma in an HIV+ man: the spectrum of HHV-8+ lymphoproliferative disorders expands. Am J Surg Pathol. 2007;31(9):1439–45.
Gonzalez-Farre B, Martinez D, Lopez-Guerra M, Xipell M, Monclus E, Rovira J, et al. HHV8-related lymphoid proliferations: a broad spectrum of lesions from reactive lymphoid hyperplasia to overt lymphoma. Mod Pathol. 2017;30(5):745–60.
Ferry JA, Sohani AR, Longtine JA, Schwartz RA, Harris NL. HHV8-positive, EBV positive Hodgkin lymphoma-like large B-cell lymphoma and HHV8-positive intravascular large B-cell lymphoma. Mod Pathol. 2009;22(5):618–26.
Bhavsar T, Lee JC, Perner Y, Raffeld M, Xi L, Pittaluga S, et al. KSHV-associated and EBV-associated germinotropic lymphoproliferative disorder: new findings and review of the literature. Am J Surg Pathol. 2017;41(6):795–800.
Veloza L, Teixido C, Castrejon N, Climent F, Carrio A, Marginet M, et al. Clinicopathological evaluation of PD1/PD-L1 axis in post-transplant lymphoproliferative disorders: association with EBV, PD-L1 copy number alterations and outcome. Histopathology. 2019;75(6):799–812.
Shannon-Lowe C, Rickinson AB, Bell AI. Epstein-Barr virus-associated lymphomas. Philos Trans R Soc Lond Ser B Biol Sci. 2017;372(1732):20160271.
Said J, Cesarman E. Primary effusion lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues (revised 4th edition). Lyon: IARC Press; 2017. p. 323–4.
Carbone A. KSHV/HHV-8 associated Kaposi’s sarcoma in lymph nodes concurrent with Epstein-Barr virus associated Hodgkin lymphoma. J Clin Pathol. 2005;58(6):626–8.
Girndt M, Sester U, Sester M, Kaul H, Köhler H. Impaired cellular immune function in patients with end-stage renal failure. Nephrol Dial Transpl. 1999;14(12):2807–10.
Chen BJ, Chapuy B, Ouyang J, Sun HH, Roemer MG, Xu ML, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus associated malignancies. Clin Cancer Res. 2013;19(13):3462–73.
Guerrero C, Jain T, Kelemen K. HHV-8-associated lymphoproliferative disorders and pathogenesis in an HIV-positive patient. Case Rep Hematol. 2019;2019:4536157.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Sanchez, S., Veloza, L., Wang, L. et al. HHV8-positive, EBV-positive Hodgkin lymphoma-like large B cell lymphoma: expanding the spectrum of HHV8 and EBV-associated lymphoproliferative disorders. Int J Hematol 112, 734–740 (2020). https://doi.org/10.1007/s12185-020-02897-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-020-02897-8