Abstract
Headspace-gas-chromatography ion-mobility spectrometry (HS-GC-IMS) proved the diversity of volatile chlorinated compounds (VCCs) in frying oil in this work. First, the VCCs were obtained by headspace by heating the frying oil at 80 °C for 30 min. Then, those compounds were separated by GC capillary column in the first dimension and by IMS in the second dimension, respectively. And at last, those compounds were detected in negative ion mode for non-targeting. The study results indicated that VCCs' formation depends on the contents of NaCl and water, heating temperature and time, and the types of oil. The refining process does not affect the detection of VCCs, indicating the durability of such targets as indicators for assessing deep-frying oil. Using HS-GC-IMS, the VCCs were detected to evaluate 16 authentic refined deep-frying oils from the market with an accuracy of 100%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frying, as a primary means of food processing, can be traced back to 1600 BC. This ancient cooking method combines heat dehydration and food cooking from the surface to the interior with oil as the heat transfer medium. It is widely used in factory production and home cooking of food. The edible oil used for frying is called frying oil, a source of energy and essential fatty acids, and serves as a carrier of fat-soluble vitamins (Park & Kim, 2016). Fried foods are popular because of their rapid and easy production.
To reduce expenses, people tend to use oil for frying repeatedly. Repeated use of this oil has become common due to the low level of awareness among the public about the harmful effect (Park & Kim, 2016). However, in the process of repeated frying with high temperature, the chlorine-related compounds, such as 3-monochloropropane-1,2-diol (3-MCPD) ester and 1,3-dichloropropan-2-ol (1,3-DCP) ester, will be produced. The 3-MCPD ester can release highly toxic-free 3-MCPD in animals to cause affections in the kidneys, blood, and sperm, which concerned by researchers (Franke et al., 2009; Matthäus et al., 2011; Stadler, 2015; Wong et al., 2017a, 2017b; Zhou et al., 2014). The free 3-MCPD also directly exists in edible oil in a small amount (Svejkovská et al., 2018). The determination method of such trace small molecular chlorinated compounds mainly based on gas chromatography-mass spectrometry (GC-MS) and gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS) (Genualdi et al., 2017; Kim et al., 2015; Küsters et al., 2010; Yang et al., 2018). However, these MS methods pose technical problems, including low m/z fragmentation patterns, expensive instrumentation, and high running costs. The molecular imprinting method was proposed to overcome these disadvantages (Fang et al., 2019).
Abundant products could be produced due to the oxidation and hydrolysis of oil during frying (Nayak et al., 2016). The chlorine can react with triacylglycerol to form the chloropropanol esters and act as repeated frying oil indicators (Zhou et al., 2014). We inferred that chlorine could react with the cleavages of triacylglycerol to form related VCCs. These VCCs predictably could also be used as indicators of repeated frying oil. However, the sensitive detection of these VCCs may also face the dilemmas like the detection of free 3-MCPD. And so far, the lack of information on these VCCs may be mainly due to the lack of sensitive detection methods, which may cause people to ignore these indicators to evaluate frying oil subjectively.
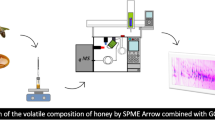
HS-GC-IMS is very similar to gas chromatography-time-of-flight mass spectrometry but works under atmospheric pressure, consisting of drift-time ion-mobility spectrometry coupled with headspace capillary gas chromatography. After the complex mixture is pretreated by incubation, the headspace components are extracted and injected into the sample inlet by a gas-tight sampler, following the GC’s first dimension separation into the ionization reaction zone of IMS. Then, analytes are soft chemical ionized. These ionized analytes enter the drift region through the periodically opened ion gate under the electric field force’s action. During the continuous collision with the countercurrent neutral drift gas molecules, ionized analytes with separate intrinsic collision cross section (CCS) values realize the second dimension.
HS-GC-IMS solves the disadvantages of low efficiency of GC and low resolution of IMS simultaneously. Taking advantage of the ultra-sensitive detection of gas phase compounds by IMS(Allafchian et al., 2016; Cohen et al., 2015; Ewing et al., 2001; Gaik et al., 2017; Holopainen et al., 2013; Jafari et al., 2012; Kalhor et al., 2016a, 2016b; Kalhor et al., 2016a, 2016b; Karpas, 2013; Langejuergen et al., 2014; Ruzsanyi et al., 2012), the HS-GC-IMS technique demonstrated as a reliable tool for the recognition of different types of edible oils, including classification (Chen et al., 2019a, 2019b; Contreras et al., 2020; Contreras et al, 2019a; Contreras et al, 2019b; Gerhardt et al., 2019), shelf life (Garrido-Delgado et al., 2015), autoxidation(Tzschoppe et al., 2016), adulteration (Othman et al., 2019; Tian et al., 2019), refining degree (Chen et al., 2019a, 2019b), and so on.
Some criminals would refine the deep-frying oil to make its relevant indicators reach the standard, causing it impossible to identify by traditional detection methods. In this study, we proposed an HS-GC-IMS device to detect the sort of VCCs in deep-frying oil for non-targeting, even if the acid and peroxide value of these oils qualified by refining.
Materials and Methods
Samples Collection and Deep Oil Frying Simulating Experiments
The food regulatory agency provided authentic deep-frying oils. We purchased other qualified oil in the supermarket.
Deep oil frying simulating experiments are similar to Zhou’s method (Zhou et al., 2014), which was conducted using an electric deep fryer, and the specified temperature chosen in this study can be reached in 10 min. Briefly, palm oil, NaCl, and water were weighed separately and then mixed by an electric mixer, after that sealed with a cover tightly, and then heated at temperatures ranging from 100 to 200 °C.
We examined the effect of NaCl content on the formation of VCCs by systems consisting of palm oil (100 g), H2O (15%), and NaCl (1, 2, 5, 10, and 20% with respect to the palm oil) at 160 °C for 4 h.
We examined the effect of water content on the formation of VCCs by systems consisted of palm oil (100 g), NaCl (5%), and H2O (1, 2, 4, 8, 12, and 15% with respect to the palm oil) at 160 °C for 4 h.
We examined the effect of heating temperature on the formation of VCCs by a system consisted of palm oil (100 g), NaCl (5%), and H2O (15%) at different temperatures (100, 160, 180, and 200 °C) for 1 h.
We examined the effect of heating time on the formation of VCCs by a system consisted of palm oil (100 g), NaCl (5 %), and H2O (4 %) at 160 °C for different times (from 0 to 48 h).
We examined the effect of types of oil on the formation of VCCs by systems consisted of a kind of edible oil (sunflower seed oil, rice oil, flaxseed oil, peanut oil, blending oil, grape seed oil, soybean oil, corn oil, rapeseed oil, and palm oil, each 100 g), NaCl (5%), and H2O (15%) at 160 °C for 2 h.
The actual deep-frying oil samples were refined as follows: the frying oil reacted with 85% phosphoric acid for 40 min and 30% sodium hydroxide for 10 min at 75 ℃, respectively, washed with 5~6% distilled water for 3 min, centrifuged at 4000 r/min for 5 min, vacuumed to 0.1 MPa at 105 ℃ until the oil was transparent, and decolorized at 110 ℃ for 30 min with 3% active clay, then deodorized at 0.1 MPa at 220 ℃ for 30 min to obtain refined frying oil. The acid and peroxide value of the refined deep-frying oils meet the relevant regulations.
We stored all samples at 4 °C prior to use.
Optimization of HS-GC-IMS Parameters Experiments
To examine the effect of incubation temperature on the detection of VCCs, we introduced a deep-frying oil (1 g) into a 20-mL headspace vial and subsequently incubated for 20 min at different temperatures (from 60 to 100 °C).
To examine the effect of incubation time on the detection of VCCs, we introduced a deep-frying oil (1 g) into a 20-mL headspace vial and subsequently incubated at 80 °C for different times (from 10 to 60 min).
We examined the negative effect of the optimal incubation temperature and time on the formation of VCCs by a system consisted of palm oil (100 g), NaCl (5 %), and H2O (4 %) at 80 °C for 30 min.
HS-GC-IMS Data Acquisition
We used a FlavourSpec@ HS-GC-IMS instrument (G.A.S., Germany) and a 60-position headspace autosampler (Combi Pal, CTC Analytics AG, Switzerland) for 20-mL HS glass vials equipped with an incubator, agitator, and a gas-tight syringe (1 mL, Hamilton, Switzerland) to analyze the VCCs in negative ion mode for non-targeting.
Volatile compounds were separated using a fused silica 15 m × 0.54 mm ID capillary column with a non-polar SE-54 stationary phase (5% diphenyl, 94% dimethyl, and 1% vinylpolysiloxane) of 1.0 μm film thickness (CS-Chromatography Service GmbH, Germany), at a constant temperature of 65 °C (± 0.1 °C). The initial carrier gas (N2, with 99.999% purity) flow rate was 3 mL/min and held for 10 min. Next, the carrier gas flow rate increased to 40 mL/min within 10 min and increased to 150 mL/min within 2 min and held for 8 min. An IMS electric field strength of 500 V/cm and a 150 mL/min drift gas (N2, with 99.999% purity) flow were applied in a drift tube of 98 mm length and operated at 45 °C. The IMS’s radioactive ionization source was tritium 3H (β radiation) of 300 MBq with an average radiation energy of 5.68 keV. Each oil sample (1 g) was introduced into a 20-mL headspace vial and subsequently incubated at 80 °C for 30 min. Finally, 500 μL of the sample headspace was injected by a heated syringe (85 °C).
The instrument control and data evaluation were done with Laboratory Analytical Viewer (LAV) software (version 2.2.1, G.A.S., Germany).
Results and Discussion
Optimization of HS-GC-IMS Parameters
The parameters of sample incubation time and temperature were investigated to optimize the HS-GC-IMS method. The optimization of incubation temperature and time considers the following two points simultaneously: one is to obtain the best peak number and peak intensity; the other is to avoid excessive incubation temperature and time from negatively affecting the generation of VCCs.
As seen in Fig. 1, the peak number and intensity increase with the increment of incubation temperature (from 60 to 90 °C, Fig. 1A) and time (from 10 to 50 min, Fig. 1B). However, we found that the higher incubation temperature and the longer incubation time will lead to contamination of the injection needle by the oil samples in a shorter experiment period. Finally, we chose the incubation temperature and time of 80 °C and 30 min, respectively, which can provide a fantastic detection effect as the optimal headspace conditions for further experiments. And the simulation experiment showed that the chosen parameters have no adverse impact on the generation of VCCs, as seen in Fig. 1C.
The optimization of HS-GC-IMS parameters for the detection of VCCs. A The optimization of incubation temperature (from 60 to 100 ℃); B the optimization of incubation time (from 10 to 60 min); C the influence of the optimal incubation temperature and time (at 80 ℃ for 30 min) on negatively affecting the generation of VCCs
VCCs Profiling by HS-GC-IMS
This study established a method for profiling the diversity of VCCs in frying oils based on HS-GC-IMS.
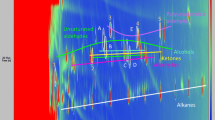
IMS can prove chlorine in volatile compounds with its inherent drift time (Karpas et al., 1993; Knighton & Grimsrud, 1992), and GC can demonstrate the diversity of volatile compounds with different retention times. Consequently, the combination of the GC retention times and the IMS drift time can identify those VCCs in deep-frying oil. As seen in Fig. 2, after the electronic exchanges with the reactant ion (Karpas et al., 1993), VCCs were detected as a series of chlorine peaks with the same IMS drift time (DT = 6.668 ms) and different GC retention times. Each dot represents a VCC, the darker the color, the higher content. The most robust peak that always appears during the entire GC separation process represents the reactant ion peak (RIP, DT = 7.409 ms).
The Necessity of Water and NaCl for the Formation of VCCs
Self-evident chlorine must be a necessary factor for the formation of VCCs. NaCl plays a critical role in the food industry, including food frying, due to its regulating taste, bacteriostasis, and color protection. So, NaCl was chosen as one of the factors to study its impact on the formation of VCCs.
In the process of food frying, the introduction of water is inevitable, for example, for the shaping of flour in the process of frying pasta. We also considered water in studying the formation of VCCs.
As shown in Fig. 3, chlorine and water are necessary factors for forming VCCs. And only when chlorines dissociate from NaCl in the water can they react with other compounds to form corresponding VCCs.
The Influence of NaCl Content on the Formation of VCCs
In general, the VCCs increase with NaCl concentration increment, as shown in Fig. 4. More NaCl (from 1 to 5%) contributes more amount of trace metals, such as iron and copper, the common pro-oxidants in NaCl for frying oil (Rossell, 2001), at the first stage. Therefore, the content of VCCs increases with NaCl’s increase at the early stage. However, with the further addition of NaCl (from 5 to 20%), the increasing trend of developing VCCs tends to relatively moderate. We can explain it as the following: the formation rate of small molecules, caused by the cleavage of triacylglycerol, is higher than that of their reaction with chlorine to form VCCs, as the results not only diluting the concentration of VCCs headspace but also excessively consuming the reactant ions to inhibit the ionization of VCCs, even though they have been separated in the GC first dimension to reduce co-elution.
The Influence of Water Content on the Formation of VCCs
The chemical changes in the frying oil are mainly based on hydrolysis and oxidation. Unlike the non-volatile hydrolysates (Nayak et al., 2016), the oxidation products of edible oil are abundant in volatile small molecular compounds, such as aldehyde, ketone, acid, and alcohol, which can be headspace after reacted with chlorine. Therefore, we inferred that water’s effect on the formation of VCCs is mainly basing on its impact on frying oil’s oxidation process. As seen in Fig. 5, there are three stages for water’s effect on the formation of VCCs, according to the previous study on the impact of water content on oil oxidation (Xu & Lu, 2008).
In the first stage, the formation of VCCs increases slowly. The little water (from 1 to 2%) can form hydrogen bonds with triglyceride hydroperoxides in the free radical reaction of frying oil oxidation, protecting it from decomposition. Simultaneously, the hydration process inhibits the catalytic ability of trace metals.
In the second stage, the formation of VCCs increases faster. The higher water (from 2 to 8%) swells fat macromolecules, exposing more oxidized sites and accelerating frying oil oxidation. As meanwhile, the higher water introduces more oxygen.
VCCs increase trends to moderate again in the third stage because a large amount of water (from 8 to 15%) dilutes the catalyst and reactants concentration.
The Influence of Heating Temperature and Time on the Formation of VCCs
The oxidation degree of oil profoundly depends on the increase in temperature. Theoretically, the VCCs content should also increase rapidly. However, the detection results do not support that hypothesis. The highest detection value was obtained by frying at 160 ℃ (Fig. 6A), but not at the higher frying temperature (180 ℃ or 200 ℃). We can explain the phenomenon for the formation rate of small molecules, caused by the cleavage of triacylglycerol, is higher than that of their reaction with chlorine to form VCCs (similar to the section “The influence of NaCl concentration on the formation of volatile chlorinated compounds”). The highest detection value obtained by frying for 8 h, but not for a longer time, shown in Fig. 6B, can also be explained in the same way.
The Influence of Refining on the Detection of VCCs
To further verify the above conjecture, we carried out the refining process by the deep-frying oil sample obtained from the food regulatory agency. The acid and peroxide value of the refined deep-frying oil meet the relevant regulations.
As seen in Fig. 7, the RIP appeared again for the consumption of the reactant ions by the excessive cleavages of triacylglycerol were eliminated considerably. The appearance of chlorine peaks indicates the refining process could not entirely remove the volatile chlorinated, the same with the 3-MCPD ester (Franke et al., 2009; Wong et al., 2017a, 2017b).
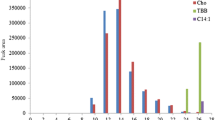
And more, we detected 16 authentic deep-frying oils in the same way with an accuracy of 100% (Fig. S1).
The Influence of the Types of Oil on the Formation of VCCs
Kinds of oils, including sunflower seed oil, rice oil, flaxseed oil, peanut oil, blending oil, grape seed oil, soybean oil, corn oil, rapeseed oil, and palm oil, were selected to study the influence of the types of oil on the formation of VCCs. The impact of the types of oil is distinct, and the highest content of VCCs produced in palm oil (Fig. S2), which agrees with the result by 3-MCPD ester (Haines et al., 2011; Pudel et al., 2011).
Conclusions
This work demonstrated a non-targeted detection of VCCs in frying oil by HS-GC-IMS. The IMS chlorine peaks, which exhibited instinct drift time in the second dimension IMS, proved the chlorine in each VCC. The retention time of the first dimension GC demonstrated the difference in the chemical properties of each VCC. The influence of the NaCl and water content, heating temperature, and time and the types of oil on the formation of VCCs were studied, respectively. By HS-GC-IMS, these VCCs can be used as indicators for evaluating deep-frying oil, even if the acid and peroxide value of it is qualified by refining. It is of great significance for effectively combating the illegal use of deep-frying oil on the market.
References
Allafchian AR, Majidian Z, Ielbeigi V, Tabrizchi M (2016) A novel method for the determination of three volatile organic compounds in exhaled breath by solid-phase microextraction-ion mobility spectrometry. Anal Bioanal Chem 408(3):839–847. https://doi.org/10.1007/s00216-015-9170-8
Chen, T., Qi, X., Chen, M., & Chen, B. (2019). Gas chromatography-ion mobility spectrometry detection of odor fingerprint as markers of rapeseed oil refined grade. J Anal Methods Chem 2019https://doi.org/10.1155/2019/3163204
Chen T, Qi X, Lu D, Chen B (2019) Gas chromatography-ion mobility spectrometric classification of vegetable oils based on digital image processing. J Food Meas Charact 13(3):1973–1979. https://doi.org/10.1007/s11694-019-00116-5
Cohen G, Rudnik DD, Laloush M, Yakir D, Karpas Z (2015) A novel method for determination of histamine in tuna fish by ion mobility spectrometry. Food Anal Methods 8(9):2376–2382. https://doi.org/10.1007/s12161-015-0129-3
del M Contreras M, Aparicio L, Arce L (2020) Usefulness of GC-IMS for rapid quantitative analysis without sample treatment: focus on ethanol, one of the potential classification markers of olive oils. Lwt 120(Novemebr 2019):108897. https://doi.org/10.1016/j.lwt.2019.108897
del M Contreras M, Arroyo-Manzanares N, Arce C, Arce L (2019a) HS-GC-IMS and chemometric data treatment for food authenticity assessment: olive oil mapping and classification through two different devices as an example. Food Control 98(2018):82–93. https://doi.org/10.1016/j.foodcont.2018.11.001
del M Contreras MM, Jurado-Campos N, Arce L, Arroyo-Manzanares N (2019b) A robustness study of calibration models for olive oil classification: targeted and non-targeted fingerprint approaches based on GC-IMS. Food Chem 288(March):315–324. https://doi.org/10.1016/j.foodchem.2019.02.104
Ewing RG, Atkinson DA, Eiceman GA, Ewing GJ (2001) A critical review of ion mobility spectrometry for the detection of explosives and explosive related compounds. Talanta 54:515–529
Fang M, Zhou L, Zhang H, Liu L, Gong ZY (2019) A molecularly imprinted polymers/carbon dots-grafted paper sensor for 3-monochloropropane-1,2-diol determination. Food Chem 274(2018):156–161. https://doi.org/10.1016/j.foodchem.2018.08.133
Franke K, Strijowski U, Fleck G, Pudel F (2009) Influence of chemical refining process and oil type on bound 3-chloro-1,2-propanediol contents in palm oil and rapeseed oil. LWT Food Sci Technol 42(10):1751–1754. https://doi.org/10.1016/j.lwt.2009.05.021
Gaik U, Sillanpää M, Witkiewicz Z, Puton J (2017) Nitrogen oxides as dopants for the detection of aromatic compounds with ion mobility spectrometry. Anal Bioanal Chem 409(12):3223–3231. https://doi.org/10.1007/s00216-017-0265-2
Garrido-Delgado R, Dobao-Prieto MM, Arce L, Aguilar J, Cumplido JL, Valcárcel M (2015) Ion mobility spectrometry versus classical physico-chemical analysis for assessing the shelf life of extra virgin olive oil according to container type and storage conditions. J Agric Food Chem 63(8):2179–2188. https://doi.org/10.1021/jf505415f
Genualdi S, Nyman P, DeJager L (2017) Simultaneous analysis of 3-MCPD and 1,3-DCP in Asian style sauces using QuEChERS extraction and gas chromatography-triple quadrupole mass spectrometry. J Agric Food Chem 65(4):981–985. https://doi.org/10.1021/acs.jafc.6b05051
Gerhardt N, Schwolow S, Rohn S, Pérez-Cacho PR, Galán-Soldevilla H, Arce L, Weller P (2019) Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem 278(November 2018):720–728. https://doi.org/10.1016/j.foodchem.2018.11.095
Haines TD, Adlaf KJ, Pierceall RM, Lee I, Venkitasubramanian P, Collison MW (2011) Direct determination of MCPD fatty acid esters and glycidyl fatty acid esters in vegetable oils by LC-TOFMS. JAOCS J Am Oil Chem Soc 88(1):1–14. https://doi.org/10.1007/s11746-010-1732-5
Holopainen S, Luukkonen V, Nousiainen M, Sillanpää M (2013) Determination of chlorophenols in water by headspace solid phase microextraction ion mobility spectrometry (HS-SPME-IMS). Talanta 114:176–182. https://doi.org/10.1016/j.talanta.2013.04.023
Jafari MT, Saraji M, Yousefi S (2012) Negative electrospray ionization ion mobility spectrometry combined with microextraction in packed syringe for direct analysis of phenoxyacid herbicides in environmental waters. J Chromatogr A 1249:41–47. https://doi.org/10.1016/j.chroma.2012.06.024
Kalhor H, Hashemipour S, Yaftian MR (2016) Ultrasound-assisted emulsification-microextraction/ion mobility spectrometry combination: application for analysis of organophosphorus pesticide residues in rice samples. Food Anal Methods 9(11):3006–3014. https://doi.org/10.1007/s12161-016-0492-8
Kalhor H, Hashemipour S, Yaftian MR, Shahdousti P (2016) Determination of carbamazepine in formulation samples using dispersive liquid–liquid microextraction method followed by ion mobility spectrometry. Int J Ion Mobil Spectrom 19(1):51–56. https://doi.org/10.1007/s12127-015-0184-x
Karpas Z (2013) Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res Int 54(1):1146–1151. https://doi.org/10.1016/j.foodres.2012.11.029
Karpas Z, Wang YF, Eiceman GA (1993) Qualitative and quantitative response characteristics of a capillary gas chromatograph/ion mobility spectrometer to halogenated compounds. Anal Chim Acta 282(1):19–31. https://doi.org/10.1016/0003-2670(93)80348-O
Kim W, Jeong YA, On J, Choi A, Lee JY, Lee JG, Lee KG, Pyo H (2015) Analysis of 3-MCPD and 1,3-DCP in various foodstuffs using GC-MS. Toxicol Res 31(3):313–319. https://doi.org/10.5487/TR.2015.31.3.313
Knighton WB, Grimsrud EP (1992) Gas-phase electron-transfer reactions between selected molecular anions and halogenated methanes. J Am Chem Soc 114(7):2336–2342. https://doi.org/10.1021/ja00033a006
Küsters M, Bimber U, Ossenbrüggen A, Reeser S, Gallitzendörfer R, Gerhartz M (2010) Rapid and simple micromethod for the simultaneous determination of 3-MCPD and 3-MCPD esters in different foodstuffs. J Agric Food Chem 58(11):6570–6577. https://doi.org/10.1021/jf100416w
Langejuergen J, Allers M, Oermann J, Kirk A, Zimmermann S (2014) High kinetic energy ion mobility spectrometer: quantitative analysis of gas mixtures with ion mobility spectrometry. Anal Chem 86(14):7023–7032. https://doi.org/10.1021/ac5011662
Xu, F., & Lu, L. X. (2008). Research progress on the oil anti-oxidation mechanism and anti-oxidation packaging of fatty food. Packaging of Fatty Food. 3–6.
Matthäus B, Pudel F, Fehling P, Vosmann K, Freudenstein A (2011) Strategies for the reduction of 3-MCPD esters and related compounds in vegetable oils. Eur J Lipid Sci Technol 113(3):380–386. https://doi.org/10.1002/ejlt.201000300
Nayak PK, Dash U, Rayaguru K, Krishnan KR (2016) Physio-Chemical changes during repeated frying of cooked oil: a review. J Food Biochem 40(3):371–390. https://doi.org/10.1111/jfbc.12215
Othman A, Goggin KA, Tahir NI, Brodrick E, Singh R, Sambanthamurthi R, Parveez GKA, Davies AN, Murad AJ, Muhammad NH, Ramli US, Murphy DJ (2019) Use of headspace-gas chromatography-ion mobility spectrometry to detect volatile fingerprints of palm fibre oil and sludge palm oil in samples of crude palm oil. BMC Res Notes 12(1):1–6. https://doi.org/10.1186/s13104-019-4263-7
Park JM, Kim JM (2016) Monitoring of used frying oils and frying times for frying chicken nuggets using peroxide value and acid value. Korean J Food Sci Anim Resour 36(5):612–616. https://doi.org/10.5851/kosfa.2016.36.5.612
Pudel F, Benecke P, Fehling P, Freudenstein A, Matthäus B, Schwaf A (2011) On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur J Lipid Sci Technol 113(3):368–373. https://doi.org/10.1002/ejlt.201000460
Rossell, J. B. (2001). Frying : improving quality.
Ruzsanyi V, Mochalski P, Schmid A, Wiesenhofer H, Klieber M, Hinterhuber H, Amann A (2012) Ion mobility spectrometry for detection of skin volatiles. J Chromatogr B Anal Technol Biomed Life Sci 911:84–92. https://doi.org/10.1016/j.jchromb.2012.10.028
Stadler RH (2015) Monochloropropane-1,2-diol esters (MCPDEs) and glycidyl esters (GEs): An update. Curr Opin Food Sci 6:12–18. https://doi.org/10.1016/j.cofs.2015.11.008
Svejkovská B, Novotný O, Divinová V, Réblová Z, Doležal M, Velíšek J (2018) Esters of 3-chloropropane-1,2-diol in foodstuffs. Czech J Food Sci 22(5):190–196. https://doi.org/10.17221/3423-cjfs
Tian L, Zeng Y, Zheng X, Chiu Y, Liu T (2019) Detection of peanut oil adulteration mixed with rapeseed oil using gas chromatography and gas chromatography–ion mobility spectrometry. Food Anal Methods 12(10):2282–2292. https://doi.org/10.1007/s12161-019-01571-y
Tzschoppe M, Haase H, Höhnisch M, Jaros D, Rohm H (2016) Using ion mobility spectrometry for screening the autoxidation of peanuts. Food Control 64:17–21. https://doi.org/10.1016/j.foodcont.2015.12.017
Wong YH, Lai OM, Abas F, Nyam KL, Nehdi IA, Muhamad H, Tan CP (2017) Factors impacting the formation of 3-MCPD esters and glycidyl esters during deep fat frying of chicken breast meat. JAOCS J Am Oil Chem Soc 94(6):759–765. https://doi.org/10.1007/s11746-017-2991-1
Wong YH, Muhamad H, Abas F, Lai OM, Nyam KL, Tan CP (2017) Effects of temperature and NaCl on the formation of 3-MCPD esters and glycidyl esters in refined, bleached and deodorized palm olein during deep-fat frying of potato chips. Food Chem 219:126–130. https://doi.org/10.1016/j.foodchem.2016.09.130
Yang S, Kwon K, Choi J, Jo CH (2018) Improvement of a GC–MS analytical method for the simultaneous detection of 3-MCPD and 1,3-DCP in food. Food Sci Biotechnol 27(3):859–866. https://doi.org/10.1007/s10068-018-0312-6
Zhou H, Jin Q, Wang X, Xu X (2014) Effects of temperature and water content on the formation of 3-chloropropane-1,2-diol fatty acid esters in palm oil under conditions simulating deep fat frying. Eur Food Res Technol 238(3):495–501. https://doi.org/10.1007/s00217-013-2126-3
Funding
This project was financially supported by the Department of science and technology of Guangdong Province (NO.2021B1111610005) and the Guangdong Provincial Medical Products Administration (NO. ZA20180016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have read and approved the final manuscript.
Conflict of Interest
Yaxiong Liu declares that he/she has no conflict of interest. Jiaxin Wen declares that he/she has no conflict of interest. Zhuoya Luo declares that he/she has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Y., Wen, J. & Luo, Z. Non-Target Detection of Diversity of Volatile Chlorine Compounds in Frying Oil and Study on the Influencing Factors of Their Formation. Food Anal. Methods 15, 940–949 (2022). https://doi.org/10.1007/s12161-021-02142-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12161-021-02142-w